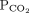TABLE 2
‘Rules of 5’ for acid-base problem-solving
| 1 Determine the arterial pH status | |||
| pH < 7.40 is acidemic, pH > 7.44 is alkalemic But a normal pH does not rule out an acid-base disorder | |||
| 2 If the arterial pH is abnormal, determine whether the primary process is respiratory, metabolic, or both | |||
| pH | 
| Bicarbonate | |
| Respiratory acidosis | Low | High | — |
| Metabolic acidosis | Low | – | Low |
| Mixed respiratory and metabolic acidosis | Low | High | Low |
| Respiratory alkalosis | High | Low | — |
| Metabolic alkalosis | High | – | High |
| Mixed respiratory and metabolic alkalosis | High | Low | High |
| 3 Calculate the anion gap | |||
| Anion gap = sodium – (chloride + bicarbonate) | |||
| If serum albumin is low, add 2.5 mmol/L to the anion gap for every 1 g the serum albumin is below normal | |||
| An anion gap > 10 mmol/L is elevated | |||
| 4 Check the degree of compensation (respiratory or metabolic) | |||
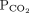 and bicarbonate should move in the same direction
and bicarbonate should move in the same direction | |||
Nominal normal levels: bicarbonate 25 mmol/L and 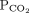 mm Hg
mm Hg | |||
In respiratory acidosis, for every 10-mm Hg increase in  , bicarbonate should increase by 1 mmol/L in the first 48 hours and 4 mmol/L afterward
, bicarbonate should increase by 1 mmol/L in the first 48 hours and 4 mmol/L afterward | |||
In metabolic acidosis, for every 1-mmol/L decrease in bicarbonate, 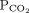 should decrease by 1.3 mm Hg
should decrease by 1.3 mm Hg | |||
In respiratory alkalosis, for every 10-mm Hg decrease in 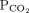 bicarbonate should decrease by 2 mmol/L in the first 48 hours and 5 mmol/L afterward
bicarbonate should decrease by 2 mmol/L in the first 48 hours and 5 mmol/L afterward | |||
In metabolic alkalosis, for every 1-mmol/L increase in bicarbonate,  may increase by 0.6 mm Hg
may increase by 0.6 mm Hg | |||
| 5 If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased | |||
| In metabolic acidosis, the anion gap should increase by the same amount that bicarbonate decreases; a difference in these two changes is called a delta gap | |||
 = partial pressure of carbon dioxide
= partial pressure of carbon dioxideBased on information in reference 1