Abstract
Introduction
The aim of the study was to evaluate the feasibility and safety of avoiding invasive mechanical ventilation (IMV) by using extracorporeal CO2 removal (ECCO2R) in patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) and acute hypercapnic respiratory failure refractory to noninvasive ventilation (NIV).
Methods
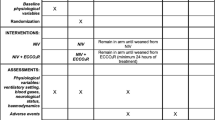
Case–control study. Patients with acute hypercapnic respiratory failure refractory to NIV being treated with a pump-driven veno-venous ECCO2R system (iLA-Activve®; Novalung, Heilbronn, Germany) were prospectively observed in five European intensive care units (ICU). Inclusion criteria were respiratory acidosis (pH ≤ 7.35, PaCO2 > 45 mmHg) with predefined criteria for endotracheal intubation (ClinicalTrials.gov NCT01784367). The historical controls were patients with acute hypercapnic respiratory failure refractory to NIV who were treated with IMV. The matching criteria were main diagnosis, age, SAPS-II score and pH.
Results
Twenty-five cases (48.0 % male, mean age 67.3 years) were matched with 25 controls. Intubation was avoided in 14 patients (56.0 %) in the ECCO2R group with a mean extracorporeal blood flow of 1.3 L/min. Seven patients were intubated because of progressive hypoxaemia and four owing to ventilatory failure despite ECCO2R and NIV. Relevant ECCO2R-associated adverse events were observed in 11 patients (44.0 %), of whom 9 (36.0 %) suffered major bleeding complications. The mean time on IMV, ICU stay and hospital stay in the case and control groups were 8.3 vs. 13.7, 28.9 vs. 24.0 and 36.9 vs. 37.0 days, respectively, and the 90-day mortality rates were 28.0 vs. 28.0 %.
Conclusions
The use of veno-venous ECCO2R to avoid invasive mechanical ventilation was successful in just over half of the cases. However, relevant ECCO2R-associated complications occurred in over one-third of cases. Despite the shorter period of IMV in the ECCO2R group there were no significant differences in length of stay or in 28- and 90-day mortality rates between the two groups. Larger, randomised studies are warranted for further assessment of the effectiveness of ECCO2R.
Similar content being viewed by others
Introduction
In patients admitted to the intensive care unit (ICU) for hypercapnic ventilatory failure, noninvasive ventilation (NIV) represents the first-line treatment to enhance alveolar ventilation, remove carbon dioxide and unload the respiratory muscle pump [1–4]. NIV has been demonstrated to reduce mortality up to almost 50 % in comparison to invasive mechanical ventilation (IMV) [5]. However, up to 50 % of these patients, especially those with severe acidosis, eventually fail initial treatment with NIV and subsequently require intubation and IMV [2, 6–9]. The transition from NIV to IMV is associated with prolonged weaning [10] and a poorer survival prognosis [2, 3, 11, 12]. The main IMV-associated complications are ventilator-induced lung injury (VILI), ventilator-associated pneumonia (VAP), ventilator-associated diaphragmatic dysfunction (VIDD), dynamic hyperinflation and a range of neurological disorders associated with prolonged sedation and immobilization [13–17].
In patients with acute on chronic ventilatory failure, ECCO2R has been proposed to avoid IMV by augmenting the effects of NIV or to facilitate early extubation [18]. However, the potential benefits of ECCO2R to avoid IMV and its complications have to be set against specific risks associated with this complex technology, especially bleeding complications. The clinical evidence for ECCO2R to primarily avoid intubation is limited to few studies, either small case series or case–control studies [19–22].
Considering the adjunctive and experimental nature of this new technology it is important to better understand in which subset of patients, at what point in the course of their disease, which type of ECCO2R and in which clinical setting the risks of IMV outweigh the risks of ECCO2R-related complications. The primary aim of this pilot study was to prospectively examine the feasibility and safety of ECCO2R to avoid IMV in patients with hypercapnic ventilatory failure refractory to NIV with a new pump-driven, veno-venous device that previously had not been evaluated prospectively in this clinical setting. Additionally these patients were compared with a matched historical control group with respect to bleeding and thromboembolic complications, length of stay and mortality.
Methods
Study design
The case–control study prospectively recruited 25 ECCO2R patients in five tertiary-level hospitals in Germany (two centres), Austria (two centres) and the Netherlands (single centre) from December 2012 to April 2015. The ECCO2R cases were matched with historical controls. The institutional ethic boards of all participating centres approved the protocol. Informed consent was obtained from all patients or their legal representatives in the ECCO2R group. The study is registered with ClinicalTrials.gov (NCT 01784367).
ECCO2R patients
Consecutive patients older than 18 years with hypercapnic ventilatory failure requiring NIV were prospectively evaluated. The nature and protocol of the study were explained to these patients and/or their legal representatives and consent was obtained on ICU admission in case the following clinical course led to failure of NIV.
Predefined combination criteria for NIV failure despite correct NIV administration were as follows: (1) no improvement or worsening of respiratory acidosis (pH ≤ 7.35, PaCO2 > 45 mmHg), (2) increased respiratory rate equal to or greater than 30 breaths/min and (3) clinical signs suggestive of ventilatory muscle pump failure, e.g. use of accessory muscles or paradoxical breathing. Moreover, single criteria for NIV failure were (1) non-compliance with mask ventilation and (2) decreased level of consciousness [Glasgow Coma Scale (GCS) <9]. The assessment of NIV failure was made by at least two attending intensivists. NIV was continued after initiation of ECCO2R at the discretion of the attending intensivist.
Study exclusion criteria were severe hypoxaemia (PaO2/FiO2 ratio <100 mmHg), pre-existing home NIV treatment, contraindications to anticoagulation, contraindication to continuation of active treatment for reasons of futility and failure to obtain consent.
The criteria for subsequent intubation despite ECCO2R with or without NIV were no improvement or worsening of the criteria mentioned above defining NIV failure with the following additional single criteria emerging during the course of ECCO2R: (1) delirium/agitation causing non-compliance and potentially leading to self-inflicted dislocation of the ECCO2R cannulas, (2) deteriorating neurological status leading to decreased level of consciousness (GCS <9) and loss of airway protective reflexes, (3) development of unmanageable copious pulmonary secretions, (4) progressive hypoxaemia (PaO2/FiO2 ratio <100 mmHg) and/or (5) haemodynamic instability requiring increasing vasopressor support.
Control patients
Control patients were identified and their data retrospectively collected from a large patient database at the principal study centre at the University Medical Center Hamburg–Eppendorf. The control patients had also been admitted to ICU with hypercapnic ventilatory failure and subsequently failed NIV treatment followed by intubation and IMV. Matching criteria for the historical controls were acute diagnosis leading to hypercapnic respiratory failure, age (±10), SAPS-II score (±6) on ICU admission and pH (±0.06) at the time of NIV failure.
ECCO2R device
The device used in the study was the pump-driven, veno-venous ECCO2R system iLA-Activve® (NOVALUNG GmbH, Heilbronn, Germany). The circuit comprises a diagonal blood pump and a low resistance heparin-coated polymethylpentene hollow-fibre membrane optimised for blood flow rates between 0.5 and 4.5 L/min with a gas exchange area of 1.3 m2 [23, 24]. A protocol of the management from cannulation to weaning of the ECCO2R can be found in the Electronic Supplementary Material (ESM).
Outcomes
In the ECCO2R group pathophysiological changes over time, the need for intubation as well as the incidence and type of ECCO2R-associated adverse events were observed and classified either as device-related (clots, air or leaks in the circuit, malfunction of the pump, rupture of the tubing) or patient-related (vascular or other injury related to cannulation and bleeding complications). According to the definition of Schulman and Kearon, bleeding episodes were considered major bleeding complications when one of the following events occurred: (1) fatal bleeding, (2) symptomatic bleeding in a critical area or organ, (3) acute bleeding causing a fall in haemoglobin level of more than 2 g/dL or (4) bleeding leading to transfusion of two or more units of packed red cells [25].
Outcomes recorded for both groups were time on mechanical ventilation, rate of tracheostomy, bleeding and thromboembolic complications, length of stay in ICU and hospital, as well as 28-day, hospital and 90-day mortality.
Statistical analysis
The software used for analyses of data was SPSS 21.0 (IBM, SPSS Statistics) and STATA 14.1 (StataCorp, College Station). Data are presented as means (with ranges) for continuous variables and frequencies (with percentages) for categorical variables. Paired t test was used for parametric analyses of within-group changes over time. For between-group comparisons of matched samples Student’s t test was used for parametric analyses and the Fisher exact test for categorical variables. In order to adjust for residual baseline differences and to increase the power of the analyses, where possible, outcomes were additionally analysed applying mixed logistic regression models with baseline covariates as fixed and pairs as random effects or Cox regression models with baseline covariates as regressors and with pairs as shared frailties. Two-sided p values below 0.05 were considered significant.
Results
Patient characteristics
A total of 25 ECCO2R patients (48.0 % male) with a mean age of 67.3 years (51.0–83.0) were prospectively enrolled in the ECCO2R group in five participating hospitals. At the principal study centre, the University Medical Center Hamburg–Eppendorf, 75 patients were screened and 16 patients (21.3 %) included into the final analyses during the full 29-month study period. A flow chart is presented in Fig. 1 in the Electronic Supplemental Material (ESM). Table 1 shows the baseline demographic, clinical and respiratory parameters of the case and control groups. Comorbidities of both groups are presented Table 1 in the ESM.
Clinical course and outcomes
The pathophysiological ventilatory changes from baseline to 24 h initiation of ECCO2R are presented in Fig. 1. Intubation was avoided in 14 out of all 25 ECCO2R patients (56.0 %). In seven patients (28.0 %) intubation was performed for progressive hypoxaemia and in four patients (16.0 %) for ventilatory failure despite ECCO2R and NIV. In five patients intubation was associated with severe secretions. In two of these five patients, intubation was caused by laryngeal oedema due to frequent bronchoscopies. In six out of seven patients progressive hypoxaemia was due to evolving infiltrates and one hypoxaemic patient was intubated for alveolar bleeding. Two patients were intubated after weaning of ECCO2R. There was no statistically significant association of the PaCO2, pH and P/F ratio pre-ECCO2R and the intubation outcome (p = 0.29, p = 0.61 and p = 0.79, respectively). Moreover, no study centre effect (Hamburg vs. other) on intubation rate was observed (p = 0.37). Table 2 in the ESM provides details of the individual clinical circumstances and indications that led to intubation in the ECCO2R patients.
The mean duration of vv-ECCO2R was 8.5 days (1.0–27.0) with a mean extracorporeal blood flow during the whole course of treatment of 1.3 L/min (0.7–1.8) using 22- or 24-Fr jugular or femoral double-lumen cannulas in the majority of patients (96.0 %). Table 3 in the ESM provides technical details regarding cannula configuration and number of membrane replacements.
The mean duration of mechanical ventilation in the ECCO2R group was 8.3 days (0–60.0) and in the control group 13.7 days (1.0–52.0; p = 0.02). Tracheostomy rates were 36.0 and 60 % in the ECCO2R and the control group, respectively (p = 0.09). Mean ICU length of stay (LOS) (28.9 vs. 24.0 days) and hospital LOS (36.9 vs. 37.0 days) did not differ significantly between the two groups. There was no significant difference in 28-day (16.0 vs. 12.0 %), hospital (24.0 vs. 12.0 %) or 90-day mortality (28.0 vs. 28.0 %) between the two groups. Details about the clinical course and outcomes are presented in Table 2.
Complications
A total of 14 major ECCO2R-associated adverse events were observed in 11 patients (44.0 %): 11 major bleeding episodes occurred in nine ECCO2R patients (36.0 %) as opposed to two major bleeding episodes in two control patients (8.0 %). The mean time from start of ECCO2R to the occurrence of the bleeding episode was 5.3 days (1.0–10.0). In three patients (12.0 %) an acute interruption of the blood flow in the vv-ECCO2R circuit occurred on day 5, 10 and 17: two patients with clotting and one patient with air detection in the circuit. Subsequently all three patients were successfully managed with NIV and did not require intubation. A total of 11 minor ECCO2R-associated adverse events were recorded and details including bleeding and thromboembolic complications of the control group are presented in Table 3. We could neither detect a significant study centre effect (Hamburg vs. other) on the complication rate (p = 0.32) nor an effect of major complications on mortality (p = 0.29).
Table 4 in the ESM shows the individual patients’ characteristics in the ECCO2R group as well as their intubation and complication occurrences. Table 5 in the ESM displays the individual ECCO2R patients’ time course of outcomes.
Discussion
In our study the application of a pump-driven, veno-venous device for ECCO2R in COPD patients with hypercapnic respiratory failure refractory to NIV prevented intubation in 56 % of cases. The avoidance of intubation in these patients, who without the application of ECCO2R would have otherwise probably been intubated, could pathophysiologically be attributed to the observed effect of significant reduction of PaCO2 and increase in arterial pH with subsequent reduction of respiratory rate as a clinical sign of unloading the ventilatory pump [19, 26].
Avoiding intubation in the majority of patients is generally in line with other recent case reports, case series and case–control studies evaluating the application of ECCO2R in patients with hypercapnic respiratory failure for this novel indication [19–22, 27, 28]. However, the degree of success rate in our study group differs from the overall success rate of 93 % in 70 patients analysed in a recent systematic review by Sklar et al. of which only 30 patients had been studied prospectively [29]. All studies differ with respect to patient characteristics, type of ECCO2R device and study design. Kluge et al. studied 21 patients who failed NIV and subsequently were treated with arterio-venous (av)-ECCO2R [21]. A selection bias caused by the retrospective design may explain the higher rate of intubation avoidance. Burki et al. and Del Sorbo et al. on the other hand prospectively evaluated nine and 25 patients respectively with two different vv-ECCO2R devices, both with maximum extracorporeal blood flows of less than 500 ml/min, and also observed high success rates [20, 22]. Del Sorbo et al. included hypercapnic patients that were “at risk of failing NIV” rather than having already failed NIV, and the intubation rate in the historic control group with “NIV-only” was only 48 % [22]. The heterogeneity of these studies in conjunction with small sample sizes may explain the differences in the magnitude of rates of intubation and makes the interpretation of different success rates difficult.
As opposed to the low-flow vv-ECCO2R devices applied in the studies by Burki et al. and Del Sorbo et al. with maximum extracorporeal blood flows of less than 500 ml/min [20, 22], the ilA-Activve® device used in our study allows blood flows up to full ECMO capacities, depending on the cannula and membrane size used. Hermann et al. recently published a retrospective case series on 12 intubated patients on IMV treated with the ilA-Activve® device for ECCO2R [24]. So far, our study is the first prospective study evaluating the ilA-Activve® device for ECCO2R. In our study population progressive hypoxaemic respiratory failure evolving after the initiation of ECCO2R and leading to intubation was observed in 28 % of patients, often associated with the development of copious secretions and in 8 % of cases with laryngeal edema secondary to frequent bronchoscopy. In retrospect, the oxygenation capacity of the ECCO2R system was not sufficient in the hypoxaemic patients and they probably would have been better managed with an endotracheal tube or with large single-lumen cannulas allowing even higher extracorporeal blood flows resulting in awake veno-venous extracorporeal membrane oxygenation (vv-ECMO). We could not demonstrate a statistically significant association of the P/F ratio pre-ECCO2R and the intubation outcome. Our results show the difficulty in predicting the clinical course at an early stage of hypercapnic respiratory failure and in selecting the right patients and the right extracorporeal configuration of ECCO2R or even ECMO to avoid intubation.
Pathophysiologically, mid-flow ECCO2R with blood flows of 1–2 L/min have been shown to more effectively remove CO2 than low-flow ECCO2R [30]. Factors such as insufficient NIV and/or membrane dysfunction in patients with severe ventilatory pump failure and progressive hypercapnia may explain failure of treatment with ECCO2R and NIV in four study patients. In one out of these four patients copious secretions may have contributed to the problem.
The rate of relevant ECCO2R-related complications (44 %) that counterbalanced the potential benefit of successful avoidance of intubation is in line with the results of previous studies on ECCO2R to avoid IMV. In their systematic review Sklar et al. found anticoagulation-related bleeding and cannulation-related vascular injury to be the two main complications of ECCO2R [29]. Corresponding with the results of this review on ECCO2R and with the evidence on ECMO-associated bleeding complications [31], bleeding also was the main type of complication in our study and significantly more frequent than in the control group (36 vs. 8 %). We could not demonstrate relevant coagulopathy in ECCO2R patients with bleeding complications. However, unmeasured clotting disorders induced by the extracorporeal surface and blood flow of our mid-flow device may have contributed to haemorrhagic complications, such as platelet dysfunction, acquired von Willebrand syndrome, hyperfibrinolysis or factor XIII deficiency [32, 33]. The higher rate of clotting of the extracorporeal circuit (30 %) observed in the study by Del Sorbo et al. may, in accordance with the principles of Virchow’s triad, in part be explained by the lower mean blood flow of 255 ml/min as opposed to 1.3 L/min in our study [22]. Moreover, by monitoring coagulation, more differentiated and individualized anticoagulation accordingly may lead to fewer bleeding and clotting complications [33]. Surprisingly, the rate of cannulation-related vascular complications was relatively low in our study in comparison to the studies by Burki et al. and Del Sorbo et al. [20, 22] despite larger catheters used in our study (22–24 Fr vs. 14–15.5 Fr). Obviating cannulation for ECCO2R altogether can be achieved in patients with acute renal and concomitant hypercapnic failure who are on low-flow continuous renal replacement therapy through Shaldon catheters (<16 Fr) by integrating an ECCO2R membrane into the renal replacement circuit [34, 35]. Larger studies, ideally with direct comparison of different ECCO2R devices, configurations, cannula sizes and resulting blood flows, are required to further evaluate the aspects mentioned above.
An important methodological limitation of our study is that the criteria used to define “NIV failure” and “indication for intubation” in the ECCO2R group left a degree of subjective clinical judgement to the attending intensivist. Furthermore, the indication for intubation in the control group could not be standardized retrospectively. The fact that by definition all control patients were intubated for NIV failure makes any interpretation of comparative effectiveness with regards to avoidance of intubation inherently difficult. Moreover, the case–control design with retrospective data acquisition in the control group cannot exclude a bias in the outcomes analysis of complications, length of stay and mortality despite statistical adjustment for potential confounding of baseline variables. Finally, statistical analysis and interpretation of our study results are further limited by the small sample size.
However, it must be stressed that the primary aim of our pilot study was to evaluate the feasibility and safety of removing CO2 extracorporeally with the pump-driven veno-venous mid-flow device investigated in order to avoid intubation. The results add more prospective data to the very limited body of current evidence and may be helpful for designing future randomized clinical trials (RCT). The relatively high rate of ECCO2R-related complications in our study will need to be prospectively compared to complications of other devices and to those of IMV. Our results suggest that in future RCTs mortality should serve as the primary outcome rather than intubation rates.
Another observation that needs to be taken into account with regards to feasibility and practicability is the fact that in 44.8 % of our study patients’ cannulation for ECCO2R took place after 2100 hours and/or on weekend days. This limits the transferability to routine clinical practice, where cannulation is not always feasible and safe during after-hours or on weekend shifts as observed by Lee et al. in patients with extracorporeal cardiopulmonary resuscitation [36]. Primary intubation of hypercapnic patients failing NIV followed by an early institution of ECCO2R would allow a more controlled cannulation with subsequent early extubation to minimize time on IMV rather than to avoid it. In addition, a short interval of IMV may identify patients who rapidly progress to hypoxaemic respiratory failure and therefore would not benefit from ECCO2R. The prospective observational pilot study by Abrams et al. has evaluated this approach [37].
In summary, the preliminary results of our pilot study show that the use of mid-flow vv-ECCO2R to avoid invasive mechanical ventilation in patients with hypercapnic ventilatory failure refractory to NIV was successful in just over half of cases. Moreover, it demonstrated the difficulty of early identification of patients that progressed to hypoxaemic respiratory failure and therefore required subsequent intubation. Relevant ECCO2R-associated complications occurred in over one-third of patients and raise concerns about the relative safety of treating patients with hypercapnic patients with mid-flow vv-ECCO2R to avoid intubation. Future, well-planned RCTs are urgently warranted to further validate the efficacy and safety of this novel strategy.
References
Nava S, Hill N (2009) Non-invasive ventilation in acute respiratory failure. Lancet 374:250–259
Chandra D, Stamm JA, Taylor B, Ramos RM, Satterwhite L, Krishnan JA et al (2012) Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med 185:152–159
Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS (2014) Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med 174:1982–1993
Schonhofer B (2015) Noninvasive ventilation in patients with persistent hypercapnia. Med Klin Intensivmed Notfmed 110:182–187
Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A et al (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333:817–822
Squadrone E, Frigerio P, Fogliati C, Gregoretti C, Conti G, Antonelli M et al (2004) Noninvasive vs invasive ventilation in COPD patients with severe acute respiratory failure deemed to require ventilatory assistance. Intensive Care Med 30:1303–1310
Confalonieri M, Garuti G, Cattaruzza MS, Osborn JF, Antonelli M, Conti G et al (2005) A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J 25:348–355
Quinnell TG, Pilsworth S, Shneerson JM, Smith IE (2006) Prolonged invasive ventilation following acute ventilatory failure in COPD: weaning results, survival, and the role of noninvasive ventilation. Chest 129:133–139
Conti G, Antonelli M, Navalesi P, Rocco M, Bufi M, Spadetta G et al (2002) Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med 28:1701–1707
Schonhofer B, Euteneuer S, Nava S, Suchi S, Kohler D (2002) Survival of mechanically ventilated patients admitted to a specialised weaning centre. Intensive Care Med 28:908–916
Hajizadeh N, Goldfeld K, Crothers K (2015) What happens to patients with COPD with long-term oxygen treatment who receive mechanical ventilation for COPD exacerbation? A 1-year retrospective follow-up study. Thorax 70:294–296
Stefan MS, Nathanson BH, Higgins TL, Steingrub JS, Lagu T, Rothberg MB et al (2015) Comparative effectiveness of noninvasive and invasive ventilation in critically ill patients with acute exacerbation of chronic obstructive pulmonary disease. Crit Care Med 43:1386–1394
Tremblay LN, Slutsky AS (2006) Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 32:24–33
Melsen WG, Rovers MM, Bonten MJ (2009) Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med 37:2709–2718
Jackson DL, Proudfoot CW, Cann KF, Walsh T (2010) A systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety. Crit Care 14:R59
Jaber S, Jung B, Matecki S, Petrof BJ (2011) Clinical review: ventilator-induced diaphragmatic dysfunction-human studies confirm animal model findings! Crit Care 15:206
Ward NS, Dushay KM (2008) Clinical concise review: mechanical ventilation of patients with chronic obstructive pulmonary disease. Crit Care Med 36:1614–1619
Braune SA, Kluge S (2013) Extracorporeal lung support in patients with chronic obstructive pulmonary disease. Minerva Anestesiol 79:934–943
Crotti S, Lissoni A, Tubiolo D, Azzari S, Tarsia P, Caspani L et al (2012) Artificial lung as an alternative to mechanical ventilation in COPD exacerbation. Eur Respir J 39:212–215
Burki NK, Mani RK, Herth FJ, Schmidt W, Teschler H, Bonin F et al (2013) A novel extracorporeal CO2 removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 143:678–686
Kluge S, Braune SA, Engel M, Nierhaus A, Frings D, Ebelt H et al (2012) Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 38:1632–1639
Del Sorbo L, Pisani L, Filippini C, Fanelli V, Fasano L, Terragni P et al (2015) Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 43:120–127
Hermann A, Riss K, Schellongowski P, Bojic A, Wohlfarth P, Robak O et al (2015) A novel pump-driven veno-venous gas exchange system during extracorporeal CO2-removal. Intensive Care Med 41:1773–1780
Hermann A, Staudinger T, Bojic A, Riss K, Wohlfarth P, Robak O et al (2014) First experience with a new miniaturized pump-driven venovenous extracorporeal CO2 removal system (iLA Activve): a retrospective data analysis. ASAIO J 60:342–347
Schulman S, Kearon C (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3:692–694
Pisani L, Fasano L, Corcione N, Comellini V, Guerrieri A, Ranieri MV et al (2015) Effects of extracorporeal CO2 removal on inspiratory effort and respiratory pattern in patients who fail weaning from mechanical ventilation. Am J Respir Crit Care Med 192:1392–1394
Mani RK, Schmidt W, Lund LW, Herth FJ (2013) Respiratory dialysis for avoidance of intubation in acute exacerbation of COPD. ASAIO J 59:675–678
Spinelli E, Crotti S, Zachetti L, Bottino N, Berto V, Russo R et al (2013) Effect of extracorporeal CO2 removal on respiratory rate in spontaneously breathing patients with chronic obstructive pulmonary disease exacerbation. Crit Care 17(Suppl 2):P128
Sklar MC, Beloncle F, Katsios CM, Brochard L, Friedrich JO (2015) Extracorporeal carbon dioxide removal in patients with chronic obstructive pulmonary disease: a systematic review. Intensive Care Med 41:1752–1762
Karagiannidis C, Kampe KA, Sipmann FS, Larsson A, Hedenstierna G, Windisch W et al (2014) Veno-venous extracorporeal CO2 removal for the treatment of severe respiratory acidosis: pathophysiological and technical considerations. Crit Care 18:R124
Gray BW, Haft JW, Hirsch JC, Annich GM, Hirschl RB, Bartlett RH (2015) Extracorporeal life support: experience with 2000 patients. ASAIO J 61:2–7
Gorlinger K, Bergmann L, Dirkmann D (2012) Coagulation management in patients undergoing mechanical circulatory support. Best Pract Res Clin Anaesthesiol 26:179–198
Kalbhenn J, Wittau N, Schmutz A, Zieger B, Schmidt R (2015) Identification of acquired coagulation disorders and effects of target-controlled coagulation factor substitution on the incidence and severity of spontaneous intracranial bleeding during veno-venous ECMO therapy. Perfusion 30:675–682
John S, Willam C (2015) Lung and kidney failure: pathogenesis, interactions, and therapy. Med Klin Intensivmed Notfmed 110:452–458
Forster C, Schriewer J, John S, Eckardt KU, Willam C (2013) Low-flow CO2 removal integrated into a renal-replacement circuit can reduce acidosis and decrease vasopressor requirements. Crit Care 17:R154
Lee DS, Chung CR, Jeon K, Park CM, Suh GY, Song YB et al (2015) Survival after extracorporeal cardiopulmonary resuscitation on weekends in comparison with weekdays. Ann Thorac Surg 101:133–140
Abrams DC, Brenner K, Burkart KM, Agerstrand CL, Thomashow BM, Bacchetta M et al (2013) Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 10:307–314
Acknowledgments
We thank all involved ICU staff of the participating centres for their commitment and effort, without whom the ECLAIR study could not have been successfully completed. Our special thanks go to Birgit Füllekrug and Brigitte Singer, the ECLAIR study nurses in the Department of Intensive Care Medicine in the Medical Center Hamburg-Eppendorf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
SB, FB, TS, DF, and AN have received lecture honoraria from Novalung GmbH, Heilbronn, Germany. SK is a member of the advisory board of Novalung GmbH and therefore has received advisor honoraria. All other authors declare that they have no conflicts of interest.
Funding
NOVALUNG GmbH (Heilbronn, Germany) provided all components of the extracorporeal circuit (iLA-Activve®) including cannulas. The company did not have any role in study design, data collection, data analysis, data interpretation, preparing the report and any decision about its publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Braune, S., Sieweke, A., Brettner, F. et al. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case–control study. Intensive Care Med 42, 1437–1444 (2016). https://doi.org/10.1007/s00134-016-4452-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4452-y