Abstract
Summary
Zoledronic acid provokes an inflammatory reaction, or acute phase response, in some individuals. We examined whether treatment with dexamethasone could prevent this response. A single dose of dexamethasone 4 mg, given at the time of zoledronic acid infusion, did not influence the incidence or severity of the acute phase response.
Introduction
The potent bisphosphonate zoledronic acid (ZOL) is used to treat osteoporosis, Paget’s disease, and hypercalcemia of malignancy. This medication can provoke an inflammatory reaction, known as the acute phase response (APR). We examined whether glucocorticoid treatment at the time of first exposure to ZOL prevents the development of APR.
Methods
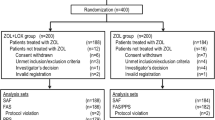
This double-blind, randomized, controlled trial assessed 40 adults receiving ZOL 5 mg intravenously for the first time. Participants received oral dexamethasone 4 mg (n = 20) or placebo (n = 20) at the time of ZOL infusion. Oral temperature was measured at baseline and three times a day for 3 days following infusion. Symptoms of APR were assessed via questionnaire at baseline then daily for 3 days and again at day 15 post-infusion. Use of rescue medications (paracetamol or ibuprofen) in the 3 days following infusion was evaluated. Primary outcome was between-group difference in temperature change from baseline.
Results
There was no significant difference in temperature change (p = 0.95) or symptom score (p = 0.42) in the 3 days following ZOL between dexamethasone and placebo recipients. Eleven (55%) in the dexamethasone group and 10 (50%) placebo recipients experienced a temperature increase of ≥1 °C (p = 0.99). Seven (35%) in the dexamethasone group and 9 (45%) in the placebo group experienced an increase in symptom score of ≥3 points (p = 0.75). Thirteen (65%) dexamethasone recipients and 12 (60%) in the placebo group required rescue medications (p = 0.99). Dexamethasone was well-tolerated.
Conclusions
A single dose of dexamethasone 4 mg does not influence the incidence or severity of APR following first exposure to ZOL.
Trial registration
ACTRN12615000794505



Similar content being viewed by others

References
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, Trial HPF (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822. doi:10.1056/NEJMoa067312
Reid IR, Lyles K, Su G, Brown JP, Walsh JP, del Pino-Montes J, Miller PD, Fraser WD, Cafoncelli S, Bucci-Rechtweg C, Hosking DJ (2011) A single infusion of zoledronic acid produces sustained remissions in Paget disease: data to 6.5 years. J Bone Miner Res 26(9):2261–2270. doi:10.1002/jbmr.438
Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, Yunus F, Bell R, Body J, Quebe-Fehling E, Seaman J (2001) Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 19(2):558–567
Grey A, Bolland M, Wattie D, Horne A, Gamble G, Reid IR (2010) Prolonged antiresorptive activity of zoledronate: a randomized, controlled trial. J Bone Miner Res 25(10):2251–2255. doi:10.1002/jbmr.103
Grey A, Bolland MJ, Horne A, Wattie D, House M, Gamble G, Reid IR (2012) Five years of anti-resorptive activity after a single dose of zoledronate—results from a randomized double-blind placebo-controlled trial. Bone 50(6):1389–1393. doi:10.1016/j.bone.2012.03.016
Wade SW, Curtis JR, Yu J, White J, Stolshek BS, Merinar C, Balasubramanian A, Kallich JD, Adams JL, Viswanathan HN (2012) Medication adherence and fracture risk among patients on bisphosphonate therapy in a large United States health plan. Bone 50(4):870–875. doi:10.1016/j.bone.2011.12.021
Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM (2010) Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 95(9):4380–4387. doi:10.1210/jc.2010-0597
Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K (2009) Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol 144(2):245–250. doi:10.1111/j.1365-2141.2008.07435.x
Anastasilakis AD, Polyzos SA, Makras P, Sakellariou GT, Bisbinas I, Gkiomisi A, Delaroudis S, Gerou S, Ballaouri I, Oikonomou D, Papapoulos SE (2012) Acute phase response following intravenous zoledronate in postmenopausal women with low bone mass. Bone 50(5):1130–1134. doi:10.1016/j.bone.2012.02.006
Welton JL, Morgan MP, Marti S, Stone MD, Moser B, Sewell AK, Turton J, Eberl M (2013) Monocytes and gammadelta T cells control the acute-phase response to intravenous zoledronate: insights from a phase IV safety trial. J Bone Miner Res 28(3):464–471. doi:10.1002/jbmr.1797
Dicuonzo G, Vincenzi B, Santini D, Avvisati G, Rocci L, Battistoni F, Gavasci M, Borzomati D, Coppola R, Tonini G (2003) Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interf Cytokine Res 23(11):649–654. doi:10.1089/107999003322558782
Bertoldo F, Pancheri S, Zenari S, Boldini S, Giovanazzi B, Zanatta M, Valenti MT, Dalle Carbonare L, Lo Cascio V (2010) Serum 25-hydroxyvitamin D levels modulate the acute-phase response associated with the first nitrogen-containing bisphosphonate infusion. J Bone Miner Res 25(3):447–454. doi:10.1359/jbmr.090819
Silverman SL, Kriegman A, Goncalves J, Kianifard F, Carlson T, Leary E (2011) Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int 22(8):2337–2345. doi:10.1007/s00198-010-1448-2
Makras P, Anastasilakis AD, Polyzos SA, Bisbinas I, Sakellariou GT, Papapoulos SE (2011) No effect of rosuvastatin in the zoledronate-induced acute-phase response. Calcif Tissue Int 88(5):402–408. doi:10.1007/s00223-011-9468-2
Rossini M, Adami S, Viapiana O, Tripi G, Zanotti R, Ortolani R, Vella A, Troplini S, Gatti D (2013) Acute phase response after zoledronic acid is associated with long-term effects on white blood cells. Calcif Tissue Int 93(3):249–252. doi:10.1007/s00223-013-9750-6
Wark JD, Bensen W, Recknor C, Ryabitseva O, Chiodo J 3rd, Mesenbrink P, de Villiers TJ (2012) Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int 23(2):503–512. doi:10.1007/s00198-011-1563-8
Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 353(16):1711–1723. doi:10.1056/NEJMra050541
Chen CH, Lu YM, Lin SY, Chen JC, Huang PJ, Huang HT, MF CYCL (2013) Prevention of acute phase reaction of intravenous bisphosphonates. Osteoporos Int 24(S4):S589. doi:10.1007/s00198-013-2537-9
Spoorenberg SM, Deneer VH, Grutters JC, Pulles AE, Voorn GP, Rijkers GT, Bos WJ, van de Garde EM (2014) Pharmacokinetics of oral vs. intravenous dexamethasone in patients hospitalized with community-acquired pneumonia. Br J Clin Pharmacol 78(1):78–83. doi:10.1111/bcp.12295
Duggan DE, Yeh KC, Matalia N, Ditzler CA, McMahon FG (1975) Bioavailability of oral dexamethasone. Clin Pharmacol Ther 18(2):205–209
Melby JC (1977) Clinical pharmacology of systemic corticosteroids. Annu Rev Pharmacol Toxicol 17:511–527. doi:10.1146/annurev.pa.17.040177.002455
Czock D, Keller F, Rasche FM, Haussler U (2005) Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 44(1):61–98. doi:10.2165/00003088-200544010-00003
Lindholm J (2014) Cushing’s disease, pseudo-Cushing states and the dexamethasone test: a historical and critical review. Pituitary 17(4):374–380. doi:10.1007/s11102-013-0509-x
Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB Jr (1994) A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab 78(2):418–422. doi:10.1210/jcem.78.2.8106630
Rief W, Barsky AJ, Glombiewski JA, Nestoriuc Y, Glaesmer H, Braehler E (2011) Assessing general side effects in clinical trials: reference data from the general population. Pharmacoepidemiol Drug Saf 20(4):405–415. doi:10.1002/pds.2067
Machin D, Campbell M, Fayers P, Pinol A (1997) Sample size tables for clinical studies, 2nd edn. Blackwell Science, Malden, MA
Zar J (1984) Biostatistical analysis (second edition). Prentice-Hall, Englewood Cliffs, New Jersey
Chen C, Lu Y, Lin S, Chen J (2013) Prevention of acute phase reaction of IV bisphosphonates (abstract). Osteoporos Int 24(S4):595. doi:10.1007/s00198-013-2537-9
Meikle AW, Tyler FH (1977) Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med 63(2):200–207
Cassidy F, Ritchie JC, Verghese K, Carroll BJ Dexamethasone metabolism in dexamethasone suppression test suppressors and nonsuppressors. Biol Psychiatry 47(7):677–680. doi:10.1016/S0006-3223(99)00252-8
Catalano A, Morabito N, Atteritano M, Basile G, Cucinotta D, Lasco A (2012) Vitamin D reduces musculoskeletal pain after infusion of zoledronic acid for postmenopausal osteoporosis. Calcif Tissue Int 90(4):279–285. doi:10.1007/s00223-012-9577-6
Acknowledgements
The authors would like to acknowledge Dr. Michael Croxson for his helpful input during the study design.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki and was approved by the New Zealand Health and Disability Ethics Committee.
Funding
This study was funded by the Health Research Council of New Zealand. EOB is a recipient of a Doctoral Scholarship from the University of Auckland and a Helios Scholarship from the University of Calgary.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Billington, E.O., Horne, A., Gamble, G.D. et al. Effect of single-dose dexamethasone on acute phase response following zoledronic acid: a randomized controlled trial. Osteoporos Int 28, 1867–1874 (2017). https://doi.org/10.1007/s00198-017-3960-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-3960-0