Abstract
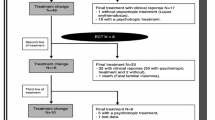
Acute catatonic syndromes occurring in the context of various medical and neuropsychiatric conditions, including schizophrenia, have been shown to respond well to benzodiazepines (BZD). However, there have been no studies specifically designed to address the BZD treatment response of persistent catatonic states. Eighteen patients with clinically stable chronic schizophrenia, who also displayed enduring catatonic features, underwent a 12-week long, random assignment, double-blind, placebo-controlled cross-over trial with lorazepam (6 mg/day). A comprehensive assessment, including the subjects’ clinical and motor (catatonic as well as drug-induced movement disorders) condition, was performed at baseline and four weekly intervals thereafter. Pre-existing medication was kept constant throughout the study. Lorazepam had no effect on the subjects’catatonic signs and symptoms, suggesting that acute and chronic catatonic syndromes associated with schizophrenic illness might have a different neurobiological basis.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 25 May 1998/Final version: 22 September 1998
Rights and permissions
About this article
Cite this article
Ungvari, G., Chiu, H., Chow, L. et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology 142, 393–398 (1999). https://doi.org/10.1007/s002130050904
Issue Date:
DOI: https://doi.org/10.1007/s002130050904