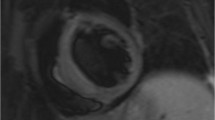
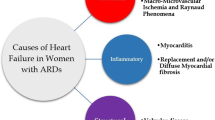
Abstract
Sudden cardiac death (SCD) is due to ventricular tachycardia/fibrillation (VT/VF) and may occur with or without any structural or functional heart disease. The presence of myocardial edema, ischemia and/or fibrosis plays a crucial role in the pathogenesis of VT/VF, irrespective of the pathophysiologic background of the disease. Specifically, in autoimmune rheumatic diseases (ARDs), various entities such as myocardial/vascular inflammation, ischemia and fibrosis may lead to VT/VF. Furthermore, autonomic dysfunction, commonly found in ARDs, may also contribute to SCD in these patients. The only non-invasive, radiation-free imaging modality that can perform functional assessment and tissue characterization is cardiovascular magnetic resonance (CMR). Due to its capability to detect and quantify edema, ischemia and fibrosis in parallel with ventricular function assessment, CMR has the great potential to identify ARD patients at high risk for VT/VF, thus influencing both cardiac and anti-rheumatic treatment and modifying perhaps the criteria for implantation of cardioverter defibrillators.


Similar content being viewed by others
References
Koplan BA, Stevenson WG (2009) Ventricular tachycardia and sudden cardiac death. Mayo Clin Proc 84(3):289–297
Migliore F, Zorzi A, Perazzolo Marra M, Iliceto S, Corrado D (2015) Myocardial edema as a substrate of electrocardiographic abnormalities and life-threatening arrhythmias in reversible ventricular dysfunction of takotsubo cardiomyopathy: imaging evidence, presumed mechanisms, and implications for therapy. Heart Rhythm 12(8):1867–1877
Mavrogeni S, Sfikakis P, Dimitroulas T, Kolovou G, Kitas GD (2014) Edema and fibrosis imaging by cardiovascular magnetic resonance: how can the experience of cardiology be best utilized in rheumatological practice? Semin Arthritis Rheum 44(1):76–85
Seferović PM1, Ristić AD, Maksimović R, Simeunović DS, Ristić GG, Radovanović G, Seferović D, Maisch B, Matucci-Cerinic M (2006) Cardiac arrhythmias and conduction disturbances in autoimmune rheumatic diseases. Rheumatology 45(Suppl 4):iv39–i42
Rosenstein ED, Zucker MJ, Kramer N (2000) Giant cell myocarditis: most fatal of autoimmune diseases. Semin Arthritis Rheum 30(1):1–16
Lazzerini PE, Bertolozzi I, Acampa M, Fulceri R, Laghi-Pasini F, Capecchi PL (2018) Torsades de pointes in patients with polymyalgia rheumatica. Curr Pharm Des 24(3):323–340
Lazzerini PE, Capecchi PL, Acampa M, Galeazzi M, Laghi-Pasini F (2014) Arrhythmic risk in rheumatoid arthritis: the driving role of systemic inflammation. Autoimmun Rev 13(9):936–944
Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE (2005) Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 52(2):402–411
Lazzerini PE, Capecchi PL, Laghi-Pasini F (2017) Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J 38(22):1717–1727
El-Sayed ZA, Mostafa GA, Aly GS, El-Shahed GS, El-Aziz MM, El-Emam SM (2009) Cardiovascular autonomic function assessed by autonomic function tests and serum autonomic neuropeptides in Egyptian children and adolescents with rheumatic diseases. Rheumatology 48(7):843–848
Metwalley KA, Hamed SA, Farghaly HS (2018) Cardiac autonomic function in children with type 1 diabetes. Eur J Pediatr 177:805–813
Videira G, Castro P, Vieira B, Filipe JP, Santos R, Azevedo E, Sá MJ, Abreu P (2016) Autonomic dysfunction in multiple sclerosis is better detected by heart rate variability and is not correlated with central autonomic network damage. J Neurol Sci 367:133–137
Jindal G, Singh S, Suri D, Rawat A, Rohit M (2012) Recurrent ventricular tachycardia in a child with juvenile dermatomyositis—an unusual association. Int J Rheum Dis 15(2):e26–e27
Dilaveris P, Pietri P, Tsiachris D, Gatzoulis K, Stefanadis C (2012) Inducible ventricular tachycardia due to dermatomyositis-related cardiomyopathy in the era of implantable cardioverter-defibrillator therapy. Circulation 125(7):967–969
Prutkin JM, Patton KK (2009) Ventricular tachycardia in a patient with inclusion-body myositis. Pacing Clin Electrophysiol 32(12):e36–e39
Lubitz SA, Goldbarg SH, Mehta D (2008) Sudden cardiac death in infiltrative cardiomyopathies: sarcoidosis, scleroderma, amyloidosis, hemachromatosis. Prog Cardiovasc Dis 51(1):58–73
Bournia VK, Tountas C, Protogerou AD, Panopoulos S, Mavrogeni S, Sfikakis PP (2018) Update on assessment and management of primary cardiac involvement in systemic sclerosis. J Scleroderma Relat Disord 3(1):53–65
Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, Gabriel S, Patel AR, Gargani L, Bombardieri S, Matucci-Cerinic M, Lombardi M, Pepe A, Aletras AH, Kolovou G, Miszalski T, van Riel P, Semb A, Gonzalez-Gay MA, Dessein P, Karpouzas G, Puntmann V, Nagel E, Bratis K, Karabela G, Stavropoulos E, Katsifis G, Koutsogeorgopoulou L, van Rossum A, Rademakers F, Pohost G, Lima JA (2016) Cardiovascular magnetic resonance in rheumatology: current status and recommendations for use. Int J Cardiol 217:135–148
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31(11):1409–1417
Abdelghani SA, Rosenthal TM, Morin DP (2016) Surface electrocardiogram predictors of sudden cardiac arrest. Ochsner J 16(3):280–289
Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Ma¨kikallio TH, Airaksinen KEJ, Myerburg RJ (2003) Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol 42:652–658
Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, Fought AJ, Topol EJ (2014) Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 127(1):95.e11–95.e17
Lown B, Wolf M (1971) Approaches to sudden death from coronary heart disease. Circulation 44(1):130–142
Mavrogeni S, Papavasiliou A, Giannakopoulou K, Markousis-Mavrogenis G, Pons MR, Karanasios E, Nikas I, Papadopoulos G, Kolovou G, Chrousos GP (2017) Oedema-fibrosis in Duchenne muscular dystrophy: role of cardiovascular magnetic resonance imaging. Eur J Clin Invest 47(12):e12843
Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A et al (2004) Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 109:2411–2416
Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE (2007) T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med 57:891–897
Friedrich MG (2010) Myocardial edema—a new clinical entity? Nat Rev Cardiol 7(5):292–296
El-Battrawy I, Lang S, Ansari U, Tülümen E, Schramm K, Fastner C, Zhou X, Hoffmann U, Borggrefe M, Akin I (2018) Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. Europace 20(5):843–850
Mavrogeni S, Bratis K, Terrovitis J, Tsagalou E, Nanas J (2012) Fulminant myocarditis. Can cardiac magnetic resonance predict evolution to heart failure? Int J Cardiol 159(2):e37–e38
Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE (2012) Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging 5:596–603
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S (2012) Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14:42
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN, Neubauer S, Moon JC (2013) Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 6:488–497
Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, Neubauer S, Moon JC, Myerson SG (2013) Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 99:932–937
Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G, Fontana M, Maestrini V, Flett AS, Robson MD, Lachmann RH, Murphy E, Mehta A, Hughes D, Neubauer S, Elliott PM, Moon JC (2013) Identification and assessment of Anderson-fabry disease by cardiovascular magnetic resonance noncontrast myocardial t1 mapping. Circ Cardiovasc Imaging 6:392–398
Scholz TD, Fleagle SR, Parrish FC, Breon T, Skorton DJ (1990) Effect of tissue fat and water content on nuclear magnetic resonance relaxation times of cardiac and skeletal muscle. Magn Reson Imaging 8:605–611
Pedersen SF, Thrysoe SA, Robich MP, Paaske WP, Ringgaard S, Botker HE, Hansen ES, Kim WY (2012) Assessment of intramyocardial hemorrhage by T1-weighted cardiovascular magnetic resonance in reperfused acute myocardial infarction. J Cardiovasc Magn Reson 14:5
Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K (2014) CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging 7(7):667–675
Nakamori S, Bui AH, Jang J, El-Rewaidy HA, Kato S, Ngo LH, Josephson ME, Manning WJ, Nezafat R (2018) Increased myocardial native T1 relaxation time in patients with nonischemic dilated cardiomyopathy with complex ventricular arrhythmia. J Magn Reson Imaging 47(3):779–786
Claridge S, Mennuni S, Jackson T, Behar JM, Porter B, Sieniewicz B, Bostock J, O’Neill M, Murgatroyd F, Gill J, Carr-White G, Chiribiri A, Razavi R, Chen Z, Rinaldi CA (2017) Substrate-dependent risk stratification for implantable cardioverter defibrillator therapies using cardiac magnetic resonance imaging: the importance of T1 mapping in nonischemic patients. J Cardiovasc Electrophysiol 28(7):785–795
Mclellan AJ, Ellims AH, Prabhu S, Voskoboinik A, Iles LM, Hare JL, Kaye DM, Macciocca I, Mariani JA, Kalman JM, Taylor AJ, Kistler PM (2016) Diffuse ventricular fibrosis on cardiac magnetic resonance imaging associates with ventricular tachycardia in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 27(5):571–580
Chen Z, Sohal M, Voigt T, Sammut E, Tobon-Gomez C, Child N, Jackson T, Shetty A, Bostock J, Cooklin M, O’Neill M, Wright M, Murgatroyd F, Gill J, Carr-White G, Chiribiri A, Schaeffter T, Razavi R, Rinaldi CA (2015) Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 imapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm 12(4):792–801
Guaricci AI, De Santis D, Rabbat MG, Pontone G (2018) Cardiac magnetic resonance imaging and primary prevention implantable cardioverter defibrillator therapy: current recommendations and future directions. J Cardiovasc Med (Hagerstown) 19(5):223–228
Saari A, Tolonen U, Pääkkö E, Suominen K, Pyhtinen J, Sotaniemi K, Myllylä V (2004) Cardiovascular autonomic dysfunction correlates with brain MRI lesion load in MS. Clin Neurophysiol 115(6):1473–1478
Thomas TO, Jefferies JL, Lorts A, Anderson JB, Gao Z, Benson DW, Hor KN, Cripe LH, Urbina EM (2015) Autonomic dysfunction: a driving force for myocardial fibrosis in young Duchenne muscular dystrophy patients? Pediatr Cardiol 36(3):561–568
Mavrogeni S, Kitas GD, Lamb HJ, Psychoyios K, Dimitroulas T, Koutsogeorgopoulou L, Boki K, Vartela V, Kolovou G, Markousis-Mavrogenis G, Kallenberg CG, Guillevin L, Vassilopoulos D (2018) Combined brain and heart magnetic resonance imaging in systemic vasculitides: fiction or real need?. Clin Exp Rheumatol 36 Suppl 111(2):152–159
Lazzerini PE, Acampa M, Capecchi PL, Fineschi I, Selvi E, Moscadelli V, Zimbone S, Gentile D, Galeazzi M, Laghi-Pasini F (2015) Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res (Hoboken) 67:332–339
Lazzerini PE, Capecchi PL, Galeazzi M, Laghi-Pasini F (2016) Biologic drugs and arrhythmic risk in chronic inflammatory arthritis: the good and the bad. Immunol Res 65:1–14
Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW. ACCF/HRS/AHA /ASE/HFSA/SCAI/SCCT/ (2013) SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 61(12):1318–1368
Huikuri HV, Castellanos A, Myerburg RJ (2001) Sudden death due to cardiac arrhythmias. N Engl J Med 345:1473–1482
Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R et al (2006) Population based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 47:1161–1166
Fishman GI, Chugh SS, DiMarco JP, Albert CM, Anderson ME, Bonow RO et al (2010) Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 122:2335–2348
Disertori M, Quintarelli S, Mazzola S, Favalli V, Narula N, Arbustini E (2013) The need to modify patient selection to improve the benefits of implantable cardioverter-defibrillator for primary prevention of sudden death in non-ischaemic dilated cardiomyopathy. Europace 15:1693–1701
Fernandes F, Ramires F, Arteaga E, Mady C (2003) Cardiac remodeling in patients with systemic sclerosis with no signs or symptoms of heart failure: an endomyocardial biopsy study. J Cardiac Fail 9(4):311–317
Koivuniemi R, Paimela L, Leirisalo-Repo M (2009) Causes of death in patients with rheumatoid arthritis from 1971 to 1991 with special reference to autopsy. Clin Rheumatol 28(12):1443–1447
Puntmann VO, D’Cruz D, Smith Z, Pastor A, Choong P, Voigt T, Carr-White G, Sangle S, Schaeffter T, Nagel E (2013) Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ Cardiovasc Imaging 6:295–301
Funding
None.
Author information
Authors and Affiliations
Contributions
SM and GK: concept and writing preparation. GMM: writing preparation. PS, LK and TD: writing on rheumatology. GK, GP and GT: writing on cardiology.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest for any of the authors.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mavrogeni, S.I., Sfikakis, P.P., Dimitroulas, T. et al. Prospects of using cardiovascular magnetic resonance in the identification of arrhythmogenic substrate in autoimmune rheumatic diseases. Rheumatol Int 38, 1615–1621 (2018). https://doi.org/10.1007/s00296-018-4110-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4110-5