Abstract
Background
Women with estrogen deficiencies can suffer from vaginal symptoms that negatively impact sexual health. This study evaluated vaginal dehydroepiandrosterone (DHEA) for alleviation of vaginal symptoms.
Methods
This three-arm randomized, controlled trial evaluated DHEA 3.25 mg and DHEA 6.5 mg, each compared to a plain moisturizer (PM) over 12 weeks, to improve the severity of vaginal dryness or dyspareunia, measured with an ordinal scale, and overall sexual health using the Female Sexual Function Index (FSFI). Postmenopausal women with a history of breast or gynecologic cancer who had completed primary treatment, had no evidence of disease, and reported at least moderate vaginal symptoms were eligible. The mean change from baseline to week 12 in the severity of vaginal dryness or dyspareunia for each DHEA dose was compared to PM and analyzed by two independent t tests using a Bonferroni correction.
Results
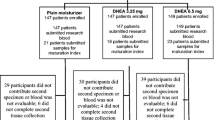
Four hundred sixty-four women were randomized. All arms reported improvement in either dryness or dyspareunia. Neither DHEA dose was statistically significantly different from PM at 12 weeks (6.25 mg, p = .08; 3.25 mg, p = 0.48), although a significant difference at 8 weeks for 6.5 mg DHEA was observed (p = 0.005). Women on the 6.5 mg arm of DHEA reported significantly better sexual health on the FSFI (p < 0.001). There were no significant differences in provider-graded toxicities and few significant differences in self-reported side effects.
Conclusion
PM and DHEA improved vaginal symptoms at 12 weeks. However, vaginal DHEA, 6.5 mg, significantly improved sexual health. Vaginal DHEA warrants further investigation in women with a history of cancer.


Similar content being viewed by others
References
Ganz PA (2005) Breast cancer, menopause, and long-term survivorship: critical issues for the 21st century. Am J Med 118(Suppl 12B):136–141
Institute of Medicine and National Research Council (2006). From Cancer Patient to Cancer Survivor: Lost in Transition. The National Academies Press, Washington DC. https://doi.org/10.17226/11468
Institute of Medicine Committee on Psychosocial Services to Cancer Patients/Families in a Community (2008) The National Academies Collection: Reports funded by National Institutes of Health. In: Adler NE, Page AEK (eds) Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. National Academies Press (US), National Academy of Sciences, Washington (DC)
Cavalheiro JA, Bittelbrunn A, Menke CH et al (2012) Sexual function and chemotherapy in postmenopausal women with breast cancer. BMC Womens Health 12:28
Malinovszky KM, Gould A, Foster E et al (2006) Quality of life and sexual function after high-dose or conventional chemotherapy for high-risk breast cancer. Br J Cancer 95(12):1626–1631
Basson R (2010) Sexual function of women with chronic illness and cancer. Women's Health (London, England) 6(3):407–429
Falk SJ, Dizon DS (2013) Sexual dysfunction in women with cancer. Fertil Steril 100(4):916–921
Kingsberg SA, Wysocki S, Magnus L et al (2013) Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women's VIews of treatment options for menopausal vaginal ChangEs) survey. J Sex Med 10(7):1790–1799
Ganz PA, Desmond KA, Belin TR, Meyerowitz BE, Rowland JH (1999) Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol: Off J Am Soc Clin Oncol 17(8):2371–2380
Carmack Taylor CL, Basen-Engquist K, Shinn EH, Bodurka DC (2004) Predictors of sexual functioning in ovarian cancer patients. J Clin Oncol: Off J Am Soc Clin Oncol 22(5):881–889
Burwell SR, Case LD, Kaelin C, Avis NE (2006) Sexual problems in younger women after breast cancer surgery. J Clin Oncol: Off J Am Soc Clin Oncol 24(18):2815–2821
Rosenberg SM, Tamimi RM, Gelber S et al (2014) Treatment-related amenorrhea and sexual functioning in young breast cancer survivors. Cancer 120(15):2264–2271
Lara LA, Useche B, Ferriani RA et al (2009) The effects of hypoestrogenism on the vaginal wall: interference with the normal sexual response. J Sex Med 6(1):30–39
Goldfarb S, Mulhall J, Nelson C, Kelvin J, Dickler M, Carter J (2013) Sexual and reproductive health in cancer survivors. Semin Oncol 40(6):726–744
North American Menopause Society (2013) The Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 20(9):888–902. https://doi.org/10.1097/GME.0b013e3182a122c2
Dew JE, Wren BG, Eden JA (2003) A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric: J Int Menopause Soc 6(1):45–52
Le Ray I, Dell'Aniello S, Bonnetain F, Azoulay L, Suissa S (2012) Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat 135(2):603–609
O'Meara ES, Rossing MA, Daling JR, Elmore JG, Barlow WE, Weiss NS (2001) Hormone replacement therapy after a diagnosis of breast cancer in relation to recurrence and mortality. J Natl Cancer Inst 93(10):754–762
Runowicz CD, Leach CR, Henry NL et al (2016) American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol: Off J Am Soc Clin Oncol 34(6):611–635
Labrie F, Martel C, Berube R et al (2013) Intravaginal prasterone (DHEA) provides local action without clinically significant changes in serum concentrations of estrogens or androgens. J Steroid Biochem Mol Biol 138:359–367
Labrie F, Archer D, Bouchard C et al (2009) Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause (New York, NY) 16(5):907–922
Labrie F, Archer D, Bouchard C et al (2009) Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause (New York, NY) 16(5):897–906
Labrie F, Archer D, Bouchard C et al (2009) Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause (New York, NY) 16(5):923–931
Pocock SJ, Simon R (1975) Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 31(1):103–115
Tan O, Bradshaw K, Carr BR (2012) Management of vulvovaginal atrophy-related sexual dysfunction in postmenopausal women: an up-to-date review. Menopause (New York, NY) 19(1):109–117
Locke DE, Decker PA, Sloan JA et al (2007) Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. J Pain Symptom Manag 34(6):628–638
Rosen R, Brown C, Heiman J et al (2000) The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 26(2):191–208
Carpenter KM, Andersen BL, Fowler JM, Maxwell GL (2009) Sexual self schema as a moderator of sexual and psychological outcomes for gynecologic cancer survivors. Arch Sex Behav 38(5):828–841
Wiegel M, Meston C, Rosen R (2005) The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther 31(1):1–20
Revicki DA, Cella D, Hays RD, Sloan JA, Lenderking WR, Aaronson NK (2006) Responsiveness and minimal important differences for patient reported outcomes. Health Qual Life Outcomes 4:70
Belsley DA, Kuh E, Welsh RE (1980) Regression diagnostics: identifying influential data and sources of collinearity. John Wiley, New York
Goetsch MF, Lim JY, Caughey AB (2015) A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol: Off J Am Soc Clin Oncol 33(30):3394–3400
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health [grant number UG1CA189823, to the Alliance for Clinical Trials in Oncology NCORP Grant], and also in part by the Public Health Service [grant numbers U10CA025224, U10CA035090, U10CA035101, U10CA035103, U10CA035113, U10CA035119, U10CA035267, U10CA035269, U10CA035415, U10CA035431, U10CA035448, U10CA037404, U10CA037417, U10CA052352, U10CA063848, U10CA063849, U10CA180790, UG1CA189863, and UG1CA189971]. This work was also supported in part by funds from a grant from the Breast Cancer Research Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by the National Cancer Institute (NCI) and the Breast Cancer Research Foundation (BCRF). However, the work is solely that of the authors and does not represent the views of the NCI or BCRF. None of the authors have a relationship that represents a conflict with either of these funding agencies. The Data and Management Center of the Alliance has full control of the primary data and if there were a question about the data that required inspection, it could be made available.
Rights and permissions
About this article
Cite this article
Barton, D.L., Sloan, J.A., Shuster, L.T. et al. Evaluating the efficacy of vaginal dehydroepiandosterone for vaginal symptoms in postmenopausal cancer survivors: NCCTG N10C1 (Alliance). Support Care Cancer 26, 643–650 (2018). https://doi.org/10.1007/s00520-017-3878-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3878-2