Abstract
Despite increased understanding of how viral infection is involved in asthma exacerbations, it is less clear which viruses are involved and to what extent they contribute to asthma exacerbations. Here, we sought to determine the prevalence of different respiratory viruses during asthma exacerbations. Systematic computerized searches of the literature up to June 2017 without language limitation were performed. The primary focus was on the prevalence of respiratory viruses, including AdV (adenovirus), BoV (bocavirus), CoV (coronavirus), CMV (cytomegalovirus), EnV (enterovirus), HSV (herpes simplex virus), IfV (influenza virus), MpV (metapneumovirus), PiV (parainfluenzavirus), RV (rhinovirus) and RSV (respiratory syncytial virus) during asthma exacerbations. We also examined the prevalence of viral infection stratified by age, geographic region, type of respiratory secretion, and detection method. Sixty articles were included in the final analysis. During asthma exacerbations, the mean prevalence of AdV, BoV, CoV, CMV, EnV, HSV, IfV, MpV, PiV, RV and RSV was 3.8%, 6.9%, 8.4%, 7.2%, 10.1%, 12.3%, 10.0%, 5.3%, 5.6%, 42.1% and 13.6%, respectively. EnV, MPV, RV and RSV were more prevalent in children, whereas AdV, BoV, CoV, IfV and PiV were more frequently present in adults. RV was the major virus detected globally, except in Africa. RV could be detected in both the upper and lower airway. Polymerase chain reaction was the most sensitive method for detecting viral infection. Our findings indicate the need to develop prophylactic polyvalent or polyvirus (including RV, EnV, IfV and RSV) vaccines that produce herd immunity and reduce the healthcare burden associated with virus-induced asthma exacerbations.
Similar content being viewed by others
Introduction
Asthma is a chronic inflammatory airway disease that is susceptible to triggering factors, such as aeroallergens, air pollutants, and viral infection [1]. Despite major advances in prevention and management, asthma remains a considerable global healthcare burden [2]. From 1990 to 2015, the crude prevalence of asthma has increased by 12.6% [3]. Currently, there are 358.2 million cases of asthma globally. Despite dramatic reductions in age-standardized mortality, 0.4 million people died from asthma in 2015 [3].
The management of asthma exacerbations, which have been consistently linked to upper and/or lower airway infections, is an important health issue [4]. Among school-age children, viral infection reportedly accounts for 80%-85% of asthma exacerbations, and viruses are more frequently isolated from symptomatic patients than from asymptomatic patients [4]. Although rhinovirus (RV) has frequently been detected in asthmatic patients [4], asthma exacerbations have also been associated with infection with other respiratory viruses, including adenovirus (AdV), bocavirus (BoV), coronavirus (CoV), cytomegalovirus (CMV), enterovirus (EnV), herpes simplex virus (HSV), influenza virus (IfV), metapneumovirus (MpV), parainfluenzavirus (PiV), and respiratory syncytial virus (RSV) [5]. Unfortunately, the rate of association of viruses with asthma exacerbations remains controversial. A hypothetical explanation might be substantial differences in study design, populations, geographic regions, detection methods, and the type of respiratory tract secretions examined.
Despite our increased understanding of the roles that viruses play in asthma exacerbations, it is less clear which viruses are involved and to what extent they contribute to asthma exacerbations in different subgroups of subjects. We conducted a systematic review of the prevalence of viral infection in patients with asthma exacerbations, with subgroup analyses to identify the determinants of variations. Our findings may offer a better overview for developing preventive interventions to minimize virus-induced asthma exacerbations.
Methods
Search strategy
Studies were searched comprehensively in EMBASE, PubMed, Cochrane Central Register of Controlled Trials and EBM Reviews-Cochrane Database of Systematic Reviews, Web of Science, Ovid and Highwire up to June 2017 (no date-of-start was specified). References were checked for additional data. We restricted our search list to publications in the English language. When the same population was used for analysis in several publications, only the largest and most complete study was included. We adopted combined keywords related to virus detection (adenovirus, bocavirus, metapneumovirus, coronavirus, Epstein-Barr virus, influenza virus, parainfluenza virus, rhinovirus, enterovirus and respiratory syncytial virus) and the outcomes of asthma exacerbations (((((((“Pulmonary Disease, Chronic Obstructive”[Mesh])) AND (“Disease Progression” [Mesh]))) OR (((copd)) AND (exacerbation)))) AND ((((“Viruses”[Mesh])) OR (respiratory viral infections)) OR (respiratory virus))) AND (((“Polymerase Chain Reaction”[Mesh])) OR (virus pcr)).
Eligibility criteria
Cross-sectional, prospective studies, cohort studies and case-control studies detailing the prevalence of respiratory virus(es) in asthma exacerbations were included. All eligible asthmatic patients were diagnosed as having viral infection based on polymerase chain reaction (PCR)/enzyme-linked immunosorbent assay (ELISA)/cell culture/direct fluorescent antibody assay (DFA)/ indirect fluorescent antibody assay (IFA), which were evaluated during exacerbations. These studies investigated asthma exacerbations in children and adults. We excluded animal studies, ex vivo and toxicological studies, summaries, commentaries and editorials, case reports and case series, duplicate publications, sample-related or laboratory-based studies, studies in intensive care settings, patients with concomitant chronic obstructive pulmonary disease, bronchiectasis or immunosuppression, non-peer reviewed articles (a potential source of bias), non-original data, nosocomial infections (hospitalization within four weeks, or delayed sample collection [>48 h after hospitalization]). Authors were contacted via e-mail if data were incomplete. Studies were excluded if no reply was obtained despite repeated contacts with the corresponding authors.
Quality score assessment
This study complied with Preferred Reporting Items for Meta-Analysis [6]. The quality of individual studies was assessed based on the revised Quality Assessment of Diagnostic Accuracy Studies-2 tool [7]. The highest total score was 12, with a higher score indicating a lower risk of bias.
Study selection and data extraction
Two independent reviewers (X.Y.Z. and Y.J.X.) screened abstracts and titles. Full texts were reviewed to determine eligibility. Disagreement was resolved by discussion. If consensus was not reached, another reviewer (W.J.G.) was consulted to vote for final decisions.
A standardized form was used for data extraction, including the main characteristics (author, year of publication, sample size, age, definition of exacerbation, quality, detection method, study design and season), primary outcome (the prevalence of viral infection during asthma exacerbations), and secondary outcomes (the prevalence of viruses in different strata). Data extraction was done by two independent reviewers (X.Y.Z. and L.F.L.). Disagreement was resolved by consultation with the third reviewer (W.J.G.).
Statistical analysis
We did a meta-analysis to obtain point estimates of the rate of asthma exacerbations associated with viral infection across the whole age spectrum globally [8]. The DerSimonian and Laird random-effect model [9] was applied because the heterogeneity among the studies was greater than 75%. The variance of raw proportions reported in each study was stabilized by using the untransformed proportion, logarithmic transformation, logit transformation, arcsine transformation or Freeman-Tukey-type arcsine square-root transformation, according to Shapiro-Wilk’s test for normality of data distribution. We also did a subgroup analysis to assess the weight of viral infection on asthma exacerbations with respect to geographic region, population, type of respiratory tract secretion examined, and detection method. If only one study was available, we calculated the 95% confidence interval (95%CI) using the Clopper-Pearson exact test. Because difference in the geographic regions, age, study population, type of respiratory tract secretion, and detection method significantly confound the determination of the prevalence of individual viruses, heterogeneity was not assessed in this study. Analyses were conducted with R software (version 2.7.2).
Results
Characteristics of included studies
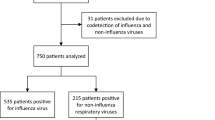
Of 438 articles relevant to our objectives, 385 were excluded. No additional studies were identified through citations within articles. Figure 1 summarizes the selection process. Sixty articles (63 studies) were analyzed to assess the association of virus infection with asthma exacerbations.
The characteristics of the patient population, study design, country, sample size, asthma definition, quality score, detection method, study design, type of respiratory tract secretion examined, and viruses involved varied substantially (E-table 1). Of the 63 eligible studies, 29 were cross sectional studies, 13 were case-control studies, 14 were cohort studies, and seven prospective studies. The studies were conducted between 1971 and 2014, with the duration varying between one month and 16 years. Asthma was diagnosed by a physician in 29 studies in which the data reported on the burden of asthma. The mean age of asthmatic patients varied among the different studies, and therefore, children and adults were dichotomized for reporting. Upper/lower airway specimens were screened by different methods, including PCR, DFA assay, IFA assay, virus culture, ELISA, rapid enzyme immunoassay and virus neutralization test.
Risk of bias of individual studies
The criteria for quality assessment and risk of bias of individual studies are summarized in E-Table 2. The mean score of quality assessment was 7.6 (range, 3 to 11). All included studies were, at least partially, prone to reporting bias.
Primary outcome
Overall, the mean prevalence (95%CI) of RV was the highest [42.1% (34.8%,49.5%)], followed by RSV [13.6% (10.1%,18.4%)], HSV [12.3% (0.7%, 23.9%)], EnV [10.1% (5.7%,17.8%)], IfV [10.0% (6.8%,14.8%)], CoV [8.4% (5.1%,13.6%)], CMV [6.9% (3.8%,10.0%)], BoV [7.2% (0.7%,75.1%)], PiV [5.6% (3.3%,9.7%)], MpV [5.3% (3.5%,8.1%)], and AdV [3.8% (1.7%,8.8%)] (Table 1, Fig. 2).
Secondary outcomes
Stratification by age
When data were stratified by age, the top five estimates of the prevalence in children were 45.7% (37.5%,53.8%) for RV, 17.7% (13.2%,23.7%) for RSV, 11.8% (6.2%,22.5%) for EnV, 8.4% (5.1%,13.6%) for CoV, and 7.4% (4.5%,12.4%) for IfV. However, in adults, RV [31.1% (18.2%,44.1%)], CoV [20.8% (12.0%,36.1%)], PiV [15.4% (7.1%,33.1%)], BoV [12.8% (3.2%,22.3%)], and IfV 19.1% (11.5%,31.7%)] were most frequently isolated in cases of asthma exacerbations. (Table 2, Fig. 3A)
The prevalence of the five most common viruses in the stratified analysis A. The prevalence of the five most common viruses when stratified by age (adults vs. children). B. The prevalence of the five most common viruses when stratified by geographic region (from top to bottom: Africa, Oceania, American, Asia, and Europe). C. The prevalence of the five most common viruses when stratified by the type of respiratory tract sample analyzed (lower vs. upper airway). D. The prevalence of the five most common viruses when stratified by method of detection (from top to bottom: culture, IFA, ELISA, DFA, and PCR). The horizontal axis represents the prevalence (%) of individual viruses. PCR, polymerase chain reaction; IFA, indirect fluorescent antibody assay; DFA, direct fluorescent antibody assay; ELISA, enzyme-linked immunosorbent assay; RV, rhinovirus; AdV, adenovirus; BoV, bocavirus; CoV, coronavirus; CV, cytomegalovirus; EnV, enterovirus; HSV, herpes simplex virus; IfV, influenza virus; MpV, metapneumovirus; PiV, parainfluenzavirus (PiV); RSV, respiratory syncytial virus
Stratification by geographic region
When stratified by geographic region, the five major viruses associated with asthma exacerbations in Europe were RV [27.4% (17.6%,37.3%)], RSV [21.5% (13.9%,33.3%)], BoV [12.4% (9.7%,15.2%)], EnV [11.7% (3.6%,38.5%)], and CoV [9.6% (3.8%,24.1%)]. In Asia, the order of prevalence for the five major viruses was RV [41.8% (18.8%,64.8%)], followed by EnV [13.3% (4.8%,37.3%)] and IfV [13.3% (4.8%,37.3%)], PiV [9.3% (3.2%,27.5%)], and RSV [8.5% (3.1%,23.4%)]. The ranking in America was RV [44.0% (31.3%,56.6%)], followed by CoV [14.2% (6.5%,31.0%)], IfV [17.5% (11.4%,26.8%)], RSV [10.6% (6.4%,17.7%)], and MpV [7.4% (5.3%,10.4%)]. RV [54.9% (36.2%, 73.6%)] was the most frequently isolated virus during asthma exacerbations in Oceania, followed by BoV [10.0% (0.0%,29.4%)], EnV [7.8% (4.7%,13.0%)], PiV [4.7% (1.4%,15.6%)], and MpV [2.6% (1.4%,5.0%)]. However, in Africa, asthma exacerbations were mainly attributable to RSV [21.5% (15.5%, 29.9%)], MpV [10.8% (6.6%, 17.7%)], PiV [4.6% (2.1%,10.1%)], AdV [3.1% (1.2%,8.1%)], and IfV [1.5 (0.4%,6.1%)]. (Table 2, Fig. 3B)
Stratification by respiratory tract secretion analyzed
Stratification based on the type of respiratory tract secretion analyzed showed that, for upper airway secretions, the top five prevalent viruses were RV [42.6% (34.8%, 50.0%)], RSV [15.1% (11.2%,20.5%)], IfV [9.9% (6.5%,14.9%)], EnV [9.5% (5.3%,17.2%)], and BoV [6.9% (3.8%,10.0%)]. However, when analyzing lower airway secretions, asthma exacerbations were attributable most frequently to RV [30.2% (17.8%, 42.5%)], followed by IfV [13.2% (6.6%, 26.3%)], AdV [3.0% (0.2%, 56.8%)], PiV [0.9% (0.1%,14.7%)] and RSV [0.9% (0.1%,14.7%)]. (Table 2, Fig. 3C)
BoV and EnV were most frequently detected in oro-/naso-pharyngeal aspirates, whereas AdV was primarily detected in induced sputum. CoV, IfV, MpV and PiV were most frequently detected in oro-/naso-pharyngeal lavage. RV and RSV were the viruses most frequently detected in spontaneous oro-/naso-pharyngeal secretions. (Table 3)
Stratification by detection method
Overall, PCR was the most sensitive technique for detecting AdV, BoV, EnV, IfV, MpV and PiV, but not for other respiratory viruses, when compared with IFA, ELISA, DFA and virus culture. ELISA yielded the highest detection rates for CoV and RSV (Table 2, Fig. 3D).
Discussion
In this study, we gathered the latest data on the prevalence of viruses detected during asthma exacerbations. Asthma exacerbations were mainly associated with RVs (42.1%), highlighting the priority for interventions to reduce the substantial healthcare burden caused by these viruses. This finding has been echoed by the high prevalence of RVs (~40%) among unselected populations residing in North America [10]. In the Netherlands, RVs were most prevalent among symptomatic children during wheezing episodes (41%) and convalescence (26%), as well as in healthy children (25%) [11]. A possible explanation is that RVs are the most common resident pathogens and are prone to cause upper airway infections (e.g., common cold). Consistently, RVs were isolated in 33.0% of pharyngeal exudate samples from Mexicans with acute upper respiratory tract infections [12].
The prevalence of viruses varied considerably among different studies. For example the range was 8-82% for RSV and 5-25% for Env) [5] (for details, see the online supplement). Intriguingly, the mean estimated prevalence of EnVs, HSV, IfV and RSV was greater than 10%, whereas other individual respiratory virus had a mean prevalence of less than 10%. In patients with other respiratory diseases, the prevalence of these viruses partially mirrored our findings. RSV was reportedly found in 12% of elderly patients with acute respiratory illnesses [13] and in 30.8% in Mexicans with acute upper respiratory tract infection [12], EnV was isolated from 37.0% of young infants with sepsis-like illnesses [14], and BoV was detected in 9.9% of nasopharyngeal aspirates from hospitalized children in Spain [15]. However, the prevalence of MpV was higher (10.6%) in Mexicans with acute upper respiratory tract infection [12], and the prevalence of AdV was also higher (15.0%) among hospitalized children in Spain [15] than that in our pooled analysis. The substantial heterogeneity of the prevalence data was probably due to differences in study design, ethnicity, geographic region, the site of sample collection, and detection methods. Therefore, direct comparisons remained challenging, and therefore subgroup analysis was done to identify possible determinants of variation.
We next stratified the prevalence data by age. EnV, MPV, RV and RSV were more prevalent in children, whereas AdV, BoV, CoV, IfV and PiV were more prevalent in adults. EnV and MPV were associated with asthma exacerbations in children [16, 17], RV were frequently associated with asthma exacerbations associated with upper respiratory tract infection in children, and RSV was commonly linked to onset of wheezing in childhood [18, 19]. The significant variation in the viruses involved probably reflected a major shift during the development of host-defense mechanisms.
The association with specific viruses varied considerably across geographic regions. RV was the most common virus in Asia, Europe, America and Oceania. RV is probably the dominant resident virus colonizing the upper and lower airways (online supplement), irrespective of meteorological factors such as temperature and humidity. RSV has been reported to be more common among populations in Africa, which could be partially related to its generally higher prevalence in low-income countries [19]. However, the reasons for the variation in the prevalence of other common respiratory viruses are not known. Further research is warranted to determine whether ethnicity-related susceptibility or meteorological factors play a significant role in shaping the prevalence of viral infections.
Consistent with literature reports (online supplement text), RV can be detected in both the upper and lower airways. For RSV, apart from the greater likelihood of initial infection of the nasopharyngeal mucosa, followed by spreading of the virus to the lower airways [20], the extensive use of nasal swabs, but not spontaneous or induced sputum for sample collection from children might also have resulted in a higher apparent prevalence in upper airway secretions. Furthermore, the higher prevalence of BoV and EnV detected in upper airway secretions might be associated with fecal-to-oral transmission [21] or transmission from oral mucosa or salivary glands [22], which would be detected more frequently in infants and toddlers.
In accordance with literature reports [23, 24], PCR was the most sensitive method for detecting viruses in asthma exacerbations. It is a simple, rapid and cost-effective technique that has become the mainstay for viral detection. Whilst virus isolation in cell culture remains the gold standard, it is time-consuming and labor-intensive, rendering it unsuitable for rapid batch detection in clinical settings [24]. Due to their limited sensitivity and specificity, other techniques such as DFA and IFA are less frequently applied clinically.
Our findings confirm that viruses of various species are closely associated with asthma exacerbations. The mechanisms are multifaceted, including 1) necrosis of airway epithelium, ciliostasis and loss of cilia [25,26,27], 2) excessive release of pro-inflammatory cytokines and chemokines [28], 3) airway hyper-responsiveness and remodeling due to epithelial injury [29], and 4) mucus hypersecretion, which impedes mucociliary clearance [30]. Unfortunately, there are few effective treatment options (e.g., immunoglobins, nebulized antivirals) for direct elimination of viral infections [31, 32]. Preemptive approaches should be directed to the prevention of viral infection, particularly in susceptible populations (e.g. children, pregnant women, the elderly, and patients with comorbidities). This has been exemplified by the vaccine development for prevention of RSV and influenza virus infection [31, 33]. Priority should be given to the most common viruses (e.g., RV, RSV, HSV, EnV, and IfV), which have caused a significant healthcare burden. Recently, a polyvalent vaccine for RV was developed in animal models [34], indicating the possibility of establishing herd immunity using polyvalent or multiple-virus vaccines in future community-based practice, which might help to achieve greater reductions in the prevalence of virus-induced asthma exacerbations.
Our results may not be readily extrapolated to all countries. For instance, documentation of the prevalence of multiple viruses in Africa is still lacking, and little is known regarding the distribution of specific viral strains. Due to the small number of studies relevant to some of our subgroup analyses (e.g., cell culture and DFA assay) even slight variations in individual studies might have had a significant effect on modified our global estimates.
We have summarized the prevalence of respiratory viruses associated with asthma exacerbations. RV, EnV and RSV are the most prevalent of these, and therefore preemptive prophylaxis with effective vaccines or novel antiviral agents is needed to minimize the healthcare burden of asthma exacerbation resulting from viral infection.
References
Castillo JR, Peters SP, Busse WW (2017) Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract 5:918–927
Masoli M, Fabian D, Holt S, Beasley R (2004) Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 59:469–478
GBD 2015 Chronic Respiratory Disease Collaborators (2017) Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study. Lancet Respir Med 5(9):691–706
Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA (1995) Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 310:1225–1229
Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, Bonini S, Bont L, Bossios A, Bousquet J, Braido F, Brusselle G, Canonica GW, Carlsen KH, Chanez P, Fokkens WJ, Garcia-Garcia M, Gjomarkaj M, Haahtela T, Holgate ST, Johnston SL, Konstantinou G, Kowalski M, Lewandowska-Polak A, Lødrup-Carlsen K, Mäkelä M, Malkusova I, Mullol J, Nieto A, Eller E, Ozdemir C, Panzner P, Popov T, Psarras S, Roumpedaki E, Rukhadze M, Stipic-Markovic A, Todo Bom A, Toskala E, van Cauwenberge P, van Drunen C, Watelet JB, Xatzipsalti M, Xepapadaki P, Zuberbier T (2011) Viruses and bacteria in acute asthma exacerbations—a GA2LEN-DARE systematic review. Allergy 66:458–468
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Sanchez-Padilla E, Grais RF, Guerin PJ, Steele AD, Burny ME, Luquero FJ (2009) Burden of disease and circulating serotypes of rotavirus infection in sub-Saharan Africa: systematic review and meta-analysis. Lancet Infect Dis 9:567–576
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Tang JW, Lam TT, Zaraket H, Lipkin WI, Drews SJ, Hatchette TF, Heraud JM, Koopmans MP, )INSPIRE investigators (2017) Global epidemiology of non-influenza RNA respiratory viruses: data gaps and a growing need for surveillance. Lancet Infect Dis S1473–3099(17):302–384
Wildenbeest JG, van der Schee MP, Hashimoto S, Benschop KS, Minnaar RP, Sprikkelman AB, Haarman EG, van Aalderen WM, Sterk PJ, Pajkrt D, Wolthers KC (2016) Prevalence of rhinoviruses in young children of an unselected birth cohort from the Netherlands. Clin Microbiol Infect 22:736.e9–736.e15
Fernandes-Matano L, Monroy-Muñoz IE, Angeles-Martínez J, Sarquiz-Martinez B, Palomec-Nava ID, Pardavé-Alejandre HD, Santos Coy-Arechavaleta A, Santacruz-Tinoco CE, González-Ibarra J, González-Bonilla CR, Muñoz-Medina JE (2017) Prevalence of non-influenza respiratory viruses in acute respiratory infection cases in Mexico. PLoS One 12:e0176298
Colosia AD, Yang J, Hillson E, Mauskopf J, Copley-Merriman C, Shinde V, Stoddard J (2017) The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: a systematic review. PLoS One 12:e0182321
de Jong EP, van den Beuken MGA, van Elzakker EPM, Wolthers KC, Sprij AJ, Lopriore E, Walther FJ, Brus F (2017) Epidemiology of sepsis-like illness in young infants: major role of enterovirus and human parechovirus. Pediatr Infect Dis J. https://doi.org/10.1097/INF.0000000000001718
Calvo C, García-García ML, Pozo F, Carballo D, Martínez-Monteserín E, Casas I (2016) Infections and coinfections by respiratory human bocavirus during eight seasons in hospitalized children. J Med Virol 88:2052–2058
Vazquez-Perez JA, Ramirez-Gonzalez JE, Moreno-Valencia Y, Hernandez-Hernandez VA, Romero-Espinoza JA, Castillejos-Lopez M, Hernandez A, Perez-Padilla R, Oropeza-Lopez LE, Escobar-Escamilla N, Gonzalez-Villa M, Alejandre-Garcia A, Regalado-Pineda J, Santillan-Doherty P, Lopez-Martínez I, Diaz-Quiñonez A, Salas-Hernandez J (2016) EV-D68 infection in children with asthma exacerbation and pneumonia in Mexico City during 2014 autumn. Influ Other Respir Viruses 10:154–160
Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, Staat MA, Iwane M, Prill MM, Williams JV, Network New Vaccine Surveillance (2013) Burden of human metapneumovirus infection in young children. N Engl J Med 368:633–643
Lukkarinen M, Koistinen A, Turunen R, Lehtinen P, Vuorinen T, Jartti T (2017) Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol 140(4):988–995
Shi T, McAllister DA, O’Brien KL, et al. RSV Global Epidemiology Network (2017) Global, regional and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390(10098):946–958
Manti S, Cuppari C, Lanzafame A, Salpietro C, Betta P, Leonardi S, Perez MK, Piedimonte G (2017) Detection of respiratory syncytial virus (RSV) at birth in a newborn with respiratory distress. Pediatr Pulmonol. https://doi.org/10.1002/ppul.23775
Iaconelli M, Divizia M, Della Libera S, Di Bonito P, La Rosa G (2016) Frequent detection and genetic diversity of human bocavirus in urban sewage samples. Food Environ Virol 8:289–295
Phyu WK, Ong KC, Wong KT (2017) Modelling person-to-person transmission in an Enterovirus A71 orally infected hamster model of hand-foot-and-mouth disease and encephalomyelitis. Emerg Microbes Infect 6:e62
Lee WM, Grindle K, Pappas T, Marshall DJ, Moser MJ, Beaty EL, Shult PA, Prudent JR, Gern JE (2007) High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol 45:2626–2634
de Crom SC, Obihara CC, de Moor RA, Veldkamp EJ, van Furth AM, Rossen JW (2013) Prospective comparison of the detection rates of human enterovirus and parechovirus RT-qPCR and viral culture in different pediatric specimens. J Clin Virol 58:449–454
van der Schans CP (2007) Bronchial mucus transport. Respir Care 52:1150–1156
Ahern W, Bird T, Court S, Gardner PS, McQuillin J (1970) Pathological changes in virus infections of the lower respiratory tract in children. J Clin Invest 23:7–18
Thornton DJ, Rousseau K, McGuckin MA (2008) Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 70:459–486
Jackson DJ, Johnston SL (2010) The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol 125:1178–1187
Leigh R, Oyelusi W, Wiehler S, Koetzler R, Zaheer RS, Newton R, Proud D (2008) Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol 121:1238–1245
Nicholas B, Skipp P, Mould R, Rennard S, Davies DE, O’Connor CD, Djukanovic R (2006) Shotgun proteomic analysis of human-induced sputum. Proteomics 6:4390–4401
Mazur NI, Martinón-Torres F, Baraldi E, Fauroux B, Greenough A, Heikkinen T, Manzoni P, Mejias A, Nair H, Papadopoulos NG, Polack FP, Ramilo O, Sharland M, Stein R, Madhi SA, Bont L, Respiratory Syncytial Virus Network (ReSViNET) (2015) Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med 3:888–900
Moss RB (2016) Enterovirus 68 infection-association with asthma. J Allergy Clin Immunol Pract 4:226–228
Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, Kabbani S, Lai L, Vassilieva EV, Skountzou I, Compans RW, Mulligan MJ, Prausnitz MR, TIV-MNP 2015 Study Group (2017) The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 390:649–658
Lee S, Nguyen MT, Currier MG, Jenkins JB, Strobert EA, Kajon AE, Madan-Lala R, Bochkov YA, Gern JE, Roy K, Lu X, Erdman DD, Spearman P, Moore ML (2016) A polyvalent inactivated rhinovirus vaccine is broadly immunogenic in rhesus macaques. Nat Commun 7:12838
Author information
Authors and Affiliations
Contributions
X. Y. Z. and W. J. G. drafted the manuscript, X. Y. Z., Y. J. X. and W. J. G. collected data, X. Y. Z. performed statistical analysis, X. Y. Z., W. J. G. and L. F. L. contributed to study conception, W. J. G. and L. F. L. provided critical review of the manuscript and approved the final submission. W. J. G. is the guarantor of the study.
Corresponding authors
Ethics declarations
Funding source
Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme 2017 (to Dr. Guan).
Conflict of interest
Xue-yan Zheng declares that she had no conflict of interest. Yan-jun Xu declares that she had no conflict of interest. Li-feng Lin declares that he had no conflict of interest. Wei-jie Guan declares that he had no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Communicated by Tim Skern.
Xue-yan Zheng and Yan-jun Xu shared co-first authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, Xy., Xu, Yj., Guan, Wj. et al. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol 163, 845–853 (2018). https://doi.org/10.1007/s00705-017-3700-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-017-3700-y