Abstract
Objectives
To compare the cost-effectiveness of first-line gefitinib, erlotinib, afatinib, and osimertinib in patients with non-small cell lung cancer (NSCLC) harbouring epidermal growth factor receptor (EGFR) mutations.
Methods
A systematic review and network meta-analysis (NMA) were conducted to compare the relative efficacy of gefitinib, erlotinib, afatinib, and osimertinib in EGFR-mutated NSCLC. To assess the cost-effectiveness of these treatments, a Markov model was developed from Dutch societal perspective. The model was based on the clinical studies included in the NMA. Incremental costs per life-year (LY) and per quality-adjusted life-year (QALY) gained were estimated. Deterministic and probabilistic sensitivity analyses (PSA) were conducted.
Results
Total discounted per patient costs for gefitinib, erlotinib, afatinib, and osimertinib were €65,889, €64,035, €69,418, and €131,997, and mean QALYs were 1.36, 1.39, 1.52, and 2.01 per patient, respectively. Erlotinib dominated gefitinib. Afatinib versus erlotinib yielded incremental costs of €27,058/LY and €41,504/QALY gained. Osimertinib resulted in €91,726/LY and €128,343/QALY gained compared to afatinib. PSA showed that gefitinib, erlotinib, afatinib, and osimertinib had 13%, 19%, 43%, and 26% probability to be cost-effective at a threshold of €80,000/QALY. A price reduction of osimertinib of 30% is required for osimertinib to be cost-effective at a threshold of €80,000/QALY.
Conclusions
Osimertinib has a better effectiveness compared to all other TKIs. However, at a Dutch threshold of €80,000/QALY, osimertinib appears not to be cost-effective.
Similar content being viewed by others
Introduction
Lung cancer is the leading cause of cancer-related mortality in The Netherlands and worldwide, with 10,346 lung cancer deaths in The Netherlands in 2014 [1]. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer with 80–85% of all cases [2]. At diagnosis, many patients with NSCLC are already in an advanced disease stage (IIIB or IV) and thus ineligible for surgical resection [3]. Platinum-based therapy is the standard first-line treatment for advanced NSCLC, which provides a median overall survival (OS) of about 8 months [4]. Nowadays, molecularly targeted agents are of high importance as treatment strategies for lung cancer patients [5]. For several cancer types, these targeted agents have come with improved outcomes, but also increased costs [6].
In NSCLC, mutations of the epidermal growth factor receptor (EGFR) play an important role in the growth and progression of tumour cells [7]. Prevalence of EGFR mutations is the highest in Asia with over 50% of all Asian patients with lung cancer type adenocarcinoma [8]. Among Dutch patients with NSCLC, the frequency of EGFR mutations is about 11% [9, 10]. Currently, three first-line EGFR tyrosine kinase inhibitors (TKIs) are used in clinical practice: gefitinib, erlotinib, and afatinib. These drugs have shown significantly improved progression-free survival (PFS) as the first-line treatment, compared to platinum-based therapy, in patients with EGFR mutation-positive (exon 19 deletion or exon 21 L858R mutation) NSCLC [11,12,13,14,15,16,17,18]. Osimertinib, a third-generation EGFR-TKI, is used as the second-line treatment in clinical practice. Recently, a randomised-controlled trial (RCT) showed a better efficacy of osimertinib compared to gefitinib and erlotinib as the first-line treatment. Moreover, clinical studies showed the ability of osimertinib to penetrate the central nervous system (CNS). This may be an advantage over the standard treatment, as it could decrease the occurrence of CNS progression [19]. Therefore, osimertinib is expected to be used as the first-line treatment in clinical practice in the near future. Clear direct evidence of the differences between gefitinib, erlotinib, afatinib, and osimertinib in terms of efficacy and toxicity is lacking as head-to-head comparisons are not available for all these TKIs. Thus, it is still uncertain whether one TKI is more favourable over the others in terms of efficacy and toxicity. Network meta-analysis (NMA) enables comparison of direct and indirect evidence across trials to synthesise the efficacy of different TKIs. Several NMAs on TKIs did not show significant differences between these drugs [20,21,22,23,24]. However, the outcomes of the NMAs differed from each other, which may be due to differences in the selection of studies and data [25]. Therefore, we built a new NMA of the efficacy of first-line gefitinib, erlotinib, afatinib, and osimertinib. In addition, lung cancer has a substantial economic burden on the health care system, with total mean hospital costs of €33,143 per patient with NSCLC in The Netherlands [26]. For NSCLC, furthermore, TKIs are administered until disease progression or unacceptable toxicity, which increases the drug acquisition costs. Nowadays, the comparative costs and effects are of growing importance for decision-makers [27]. Therefore, information on the incremental value of new treatments in terms of effects and costs is needed for medical resource optimisation. However, not only the acquisition costs of the drugs should be taken into account in the assessment of the cost-effectiveness, but also, for example, costs of adverse event management, travelling, and productivity losses [28]. Hence, we aimed to assess the cost-effectiveness of first-line gefitinib, erlotinib, afatinib, and osimertinib in patients with stage IIIB/IV NSCLC harbouring EGFR mutations (exon 19 deletion or exon 21 L858R mutation) in The Netherlands from a Dutch societal perspective.
Methods
Systematic review and network meta-analysis
A systematic search of several databases (PubMed, EMBASE, and Cochrane Library) was conducted to identify phase IIB/III RCTs of first-line EGFR-TKI (including gefitinib, erlotinib, afatinib, or osimertinib) compared to another TKI or platinum-based therapy. Search strategy and inclusion and exclusion criteria can be found in Appendix I. Reference lists of published studies were also checked as additional information. The literature review was conducted by two reviewers (MH and CU). After screening titles and abstracts and then full-text reading of the records found by the systematic review, 12 unique RCTs were included in the NMA [11,12,13,14,15,16,17, 19, 29,30,31,32]. Quality and risk of bias of the included RCTs were assessed using the Cochrane Collaboration’s tool for assessing risk of bias. According to this assessment, all RCTs were classified as having acceptable quality and low risk of bias [25]. Data on patient characteristics, interventions, comparators, and treatment effects [PFS, OS, and adverse events (AEs)] were extracted. For the NMA, the outcomes of interest were PFS and OS. Since no separate HRs of osimertinib versus gefitinib or osimertinib versus erlotinib were reported in the FLAURA trial, the HRs of PFS and OS were assumed to be the same for both comparisons. A fixed-effects network meta-analysis in WinBUGS 1.4 was built within a Bayesian framework by use of an adapted version of WinBUGS code from Dias et al. [33]. Due to the limited number of RCTs per TKI arm, heterogeneity could not be appropriately assessed. Therefore, a fixed-effect NMA was considered as appropriate. The methods of the NMA are described in more detail in Appendix I and in a previous study [34]. The results of the NMA are presented in Table 1. Osimertinib had a significantly better PFS and OS compared to gefitinib, erlotinib, and afatinib.
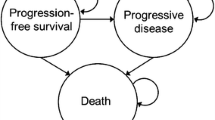
Model construction
A Markov model was constructed simulating the transition between three health states: progression-free, progression, and death, in which death was an absorbing state. A cycle length of 30 days was used for the model, which is an appropriate length given the rate at which lung cancer develops. In this model, during each cycle, patients with EGFR-mutated NSCLC move between the health states according to the transition probabilities. In each cycle, patients could remain progression-free, may progress, or die. A lifetime time horizon was used, in line with the Dutch guidelines [28], accounting for all relevant costs and effects of TKI therapies for patients with EGFR mutations. Half-cycle correction was applied to both costs and effects. Effects are expressed in life-years (LYs) gained and in quality-adjusted life-years (QALYs) gained. Outcomes are presented as incremental cost-effectiveness ratios (ICERs), i.e., incremental costs per LY gained and incremental costs per QALY gained.
Clinical effectiveness
Estimates of the clinical effectiveness in terms of pooled HRs were derived from the NMA. Since HRs only convey information on comparative effectiveness, whereas a model requires absolute estimates of PFS and OS, we used an indirect approach to estimate the transitions of patients treated with TKIs in the model. The NMA did not only include the four TKIs, but also chemotherapy. Thus, we first explored the Kaplan–Meier (KM) curves of PFS and OS for patients with EGFR mutations treated with chemotherapy from the EURTAC trial of erlotinib versus chemotherapy. According to clinical experts, the data of the chemotherapy patients in the EURTAC trial [15] were deemed as most representative for our study as patient characteristics of that trial are most similar to the Dutch patient population eligible for TKIs (i.e., Caucasian population, mainly adenocarcinoma histology, mainly stage IV NSCLC). However, as the time horizon of the model is life time, whereas the KM curves are truncated at 40 months, where 15% of the patients are still alive, it was necessary to extrapolate the KM curve using a parametric survival curve. Since we had no access to the individual patient data (IPD) of the EURTAC trial, the method of Hoyle and Henley [35] was used to recreate the IPD. Times and survival probabilities were read off from the published KM graph. Based on these survival probabilities and corresponding time and provided numbers at risk, the method of Hoyle and Henley estimated the underlying number of events and censorships in each time interval. By use of the statistical programme R, several survival distributions were fit to the recreated IPD. Based on the fit to the KM curve and the Akaike and Bayesian Information Criterion (AIC and BIC) estimates, a Weibull distribution was assessed as having the best goodness-of-fit for both PFS and OS (see Appendix II). The general Weibull equation is as follows (in which ‘t’ is time in months): \(S ( t ) = e^{ - \lambda t^{\gamma } }.\) Lambda and gamma parameters of the patients treated with chemotherapy in the EURTAC trial were used to estimate the parameters for gefitinib, erlotinib, afatinib, and osimertinib, as previously described in published studies [36, 37]. For example, the lambda parameter (scale parameter) for gefitinib was estimated by multiplying the lambda for chemotherapy by the pooled HR of gefitinib versus chemotherapy. The gamma parameter (shape parameter) was set equal to the gamma for chemotherapy. The same was done for erlotinib, afatinib, and osimertinib. These parameters were used as input to calculate the transitions of all TKIs.
For each TKI, the percentage of patients in progression-free state at each time is determined by the values of the PFS curve at that time. Similarly, the percentage of patients in the death state is determined as 1 minus the OS curve at that time. From this, the percentage of patients in the progressed state follows, as the three states together should always add up to 100%.
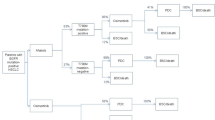
After progression on first-line gefitinib, erlotinib, or afatinib, patients were tested for T790 M mutations. Patients who were T790 M mutation-positive received the second-line osimertinib (50% of all patients) and patients who were T790 M mutation-negative were treated with pemetrexed–cisplatin [5, 38]. Patients who had progressive disease on the first-line osimertinib received the second-line pemetrexed–cisplatin treatment. Thus, the progressed health state is split into a ‘progression-free second line’ and ‘progressed second line’ health state for those patients receiving a second-line treatment. Clinical data of second-line osimertinib and pemetrexed–cisplatin were derived from the literature [39, 40]. The KM curves of second-line osimertinib and pemetrexed–cisplatin were also extrapolated by fitting various parametric functions. For both second-line PFS and OS, the exponential function was assessed as having the best fit to the KM curves of second-line osimertinib and pemetrexed–cisplatin. The survival curves of all treatment options and the estimation of the transition parameters can be found in Appendix II. After progression on the second-line osimertinib or pemetrexed-cisplatin, it was assumed that patients were treated with best supportive care (BSC) until death.
Utility weights
Health utility values reflecting the health-related quality of life in each health state were obtained from the literature [41]. The progression-free health state had the highest possible utility value while receiving TKI, with an estimated value of 0.71. This utility value was the same for all three TKI treatments. Progressive disease led to disutility for all TKIs. After progression on the first-line TKI treatment, the utility value was estimated at 0.67 (irrespective of post-progression treatment with osimertinib or pemetrexed–cisplatin) and after progression on the second-line treatment at 0.62 [41].
Disutility scores of severe adverse events (SAEs) with grades 3 or higher for the first-line gefitinib, erlotinib, afatinib, osimertinib, second-line osimertinib, and pemetrexed-cisplatin were also included in the analyses. Occurrence of SAEs was extracted from the RCTs [11,12,13,14,15,16,17,18,19, 30,31,32] and were only included when at least 1.5% of the patients experienced a certain SAE. The disutility estimates were derived from the literature. The SAEs were assumed to all occur in the first simulation cycle of that specific treatment, since the adverse events commonly appear within the first weeks after starting these treatments [42, 43]. For the future effects, a discount rate of 1.5% was applied, according to the Dutch guidelines [28]. All utility values are presented in Table 2.
Costs
Following the Dutch guideline, a societal perspective was used for the model. Table 2 shows all unit costs of gefitinib, erlotinib, afatinib, and osimertinib treatment. Costs were based on the Dutch Costing manual, the Dutch Health Care Institute, Dutch Healthcare Authority, and the literature [27, 44, 45]. All costs are in Euros, based on the average consumer price index of 2018. Future costs were discounted by a rate of 4%, according to the Dutch guidelines [28]. More details on the costs can be found in Appendix II.
Sensitivity analyses
Since the cost-effectiveness model is based on a number of assumptions, several scenario analyses were performed to test the robustness of these assumptions. In the first scenario tested, a log-logistic function instead of the Weibull function was used to estimate the survival probabilities in the model. Second, the chemotherapy patient group from another clinical trial (Lux-Lung 6) [17] was used to estimate the survival probabilities of gefitinib, erlotinib, afatinib, and osimertinib. Third, docetaxel instead of pemetrexed–cisplatin was included as the second-line treatment.
Deterministic (DSA) sensitivity analysis was performed to determine which input parameters of the model were most influential on the results of the model and to test the robustness of the model. In this DSA, the impact of varying single input parameters on the cost-effectiveness ratio while holding the others constant was assessed. If available, the 95% confidence intervals (CI) of input estimates were used for the DSA. If not, parameters were varied with ± 20% of the mean. Probabilistic sensitivity analysis (PSA) was performed by simultaneously varying all the input parameters in a Monte Carlo simulation according to pre-specified distributions. Survival parameters lambda and gamma were assumed to be bivariate normal distributed, for utilities and probabilities, a beta distribution was applied and a gamma distribution was used for costs. Standard errors of utilities and probabilities were either obtained from the literature or calculated by 10% of the mean point estimate and 20% was used for the costs. In total, 1000 simulation samples were randomly drawn from the distributions of all inputs, and each time, the model results (incremental costs and incremental effects) were recalculated. We constructed a cost-effectiveness plane that shows the base-case ICER and the uncertainty surrounding the estimated costs and effects of the pairwise comparisons. Based on the cost-effectiveness plane, a cost-effectiveness acceptability curve was constructed, which shows the probability that a treatment is cost-effective compared to the alternative, given a range of threshold ICERs [46, 47].
Results
Base-case results
Table 3 shows the incremental base-case results of the cost-effectiveness analyses. Gefitinib and erlotinib showed the lowest total discounted costs per patient and osimertinib had the highest estimated costs for patients with EGFR-mutated NSCLC. Osimertinib yielded the most effects, followed by afatinib, erlotinib, and gefitinib. Compared to gefitinib, erlotinib resulted in a QALY gain of 0.03 (and 0.03 LYs) and cost savings of €1854 per patient, indicating that erlotinib dominates gefitinib. Afatinib compared to erlotinib yielded 0.13 QALYs (and 0.20 LYs) gained and a cost increase of €5383 per patient, which resulted in an ICER of €27,058/LY and €41,504/QALY for afatinib versus erlotinib. Osimertinib yielded 0.49 QALYs (and 0.68 LYs) and €62,579 more costs relative to afatinib. Thus, an additional €91,726 per LY and €128,343 per QALY gained is spent on osimertinib compared to afatinib. The results of all other comparisons can be found in Appendix III.
Scenario analysis
Considering a Dutch threshold of €80,000/QALY, osimertinib appears not to be cost-effective (ICER of osimertinib vs. afatinib was €128,343/QALY). For osimertinib, a price reduction of 30% is required to be regarded as cost-effective (Appendix III).
Sensitivity analyses
Based on visual inspection, the Log-Logistic distribution for PFS can be regarded as a plausible alternative for the Weibull distribution. Since the Log-Logistic distribution also scored second for AIC and BIC (see Appendix II), we performed a scenario analysis using the Log-Logistic distribution to estimate the survival probabilities, which were then included into the model. This mainly resulted into lower incremental costs and a lower ICER for osimertinib compared to afatinib. In another scenario, the chemotherapy patient group from the Lux-Lung 6 trial [17] was used instead of the EURTAC trial to estimate the survival probabilities of the TKIs. This scenario resulted in lower incremental costs and QALYs, especially for the comparison of osimertinib versus afatinib. Inclusion of another second-line treatment than pemetrexed-cisplatin hardly affected the results (see Table C2 in Appendix III).
Since the comparison of osimertinib versus afatinib is most interesting (as gefitinib is dominated by erlotinib and afatinib is cost-effective compared to erlotinib), only the tornado diagram of this comparison is presented here (Fig. 1). DSA showed that the utility value of the progression-free health state seemed to be the most influential drivers. The tornado diagrams of erlotinib versus gefitinib and of afatinib versus erlotinib can be found in Appendix III.
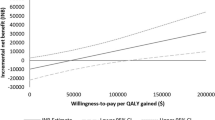
Figure 2 shows that almost 100% of the 1000 PSA iterations were in the upper right quadrant, which means that more QALYs gained at additional costs for osimertinib compared to afatinib. For afatinib versus erlotinib, about 60% of the PSA iterations were in the upper right quadrant, 20% fell within the lower right quadrant, 10% in the upper left, and another 10% was in the lower left quadrant. For erlotinib compared to gefitinib, about 30% of the iterations fell within both the lower left and upper right quadrant, and about 20% fell within both the upper left and lower right quadrant. The cost-effectiveness acceptability curves (CEAC) of all TKIs are shown in Fig. 3. At a Dutch threshold of €80,000/QALY, afatinib had the highest probability of being cost-effective (43%). Gefitinib, erlotinib, and osimertinib had a probability of 13%, 19%, and 26%, respectively, of being cost-effective at the Dutch threshold. At a threshold of €200,000/QALY, the probability of being cost-effective was 75% for osimertinib.
Discussion
To the best of our knowledge, this was the first study in The Netherlands that compared the cost-effectiveness of first-line gefitinib, erlotinib, afatinib, and osimertinib for EGFR mutation-positive (exon 19 deletion or exon 21 L858R mutation) NSCLC patients. Our study found that erlotinib dominated gefitinib. Afatinib resulted in a cost per QALY of €41,504 compared to erlotinib. Compared to afatinib treatment, osimertinib had an ICER of €128,343 per QALY gained. Thus, osimertinib was the most efficacious treatment option, followed by afatinib, erlotinib, and gefitinib, but at a high cost.
Our results are similar to the results of Aguiar et al. with ICERs of $219,874/QALY of osimertinib vs. afatinib in the US and $175,432/QALY in Brazil [48]. In a report from the Dutch Health Care Institute (ZIN), osimertinib yielded an ICER of €324,006/QALY compared to gefitinib, erlotinib, and afatinib. An ICER range from €70,847 to €324,006 was reported and the upper limit was used to calculate the required price reduction for osimerinib to be regarded as cost-effective (reduction of 55% at threshold of €80,000). The study submitted to ZIN used the effectiveness of only one trial (FLAURA trial), and thus, not all available evidence was used to estimate the effectiveness of the drugs. Utility values for progression-free health state also differed: 0.829 in the report versus 0.71 in this study [41, 49]. Since the utility values reported by the manufacturer were higher than previous reported utility values for this patient population, these values were not used in this study. When we take these aspects into account, our results would be in the order of the findings of the ZIN report. In other cost-effectiveness studies, only two TKIs were compared [50,51,52,53]. Lee et al. [51] showed incremental costs per QALY gained by erlotinib compared to gefitinib of $62,419 (incremental costs $14,061 and incremental QALY 0.23) and $41,494 per LY gained (incremental LY 0.34). These results are different from our study. This might be due to the fact that Lee et al. [51] simulated the survival probability for erlotinib based on the OS outcomes of the IPASS trial [18], because the OS results of erlotinib were still immature at that moment. In addition, more studies were included in our analyses. Ting et al. [50] analysed the cost-effectiveness of erlotinib versus afatinib and found a mean ICER of $61,809/QALY, with incremental costs $6417 and incremental QALY 0.17 [50]. These outcomes are the opposite of our results. A plausible reason might be that only the EURTAC and Lux-Lung 3 trials were used for the data of erlotinib and afatinib, while we included various trials besides these two in our network [11,12,13,14,15,16,17, 19, 29,30,31,32]. Furthermore, Ting et al. [50] have corrected the survival probabilities of erlotinib for patients with more severe disease. However, survival estimates were not corrected for other prognostic factors that were unequally distributed among the two treatments (e.g., EGFR mutation type). Correcting for only one prognostic factor could result into biased corrections. When uncorrected survival probabilities were added in the study of Ting et al., erlotinib became less expensive and survival decreased. This yielded an ICER of $534,903 for afatinib versus erlotinib (incremental costs $7494 and incremental QALY 0.014) [50].
Our results were similar to the cost-effectiveness ratios reported by Chouaid et al. [53] and the National Institute of Health and care Excellence (NICE) [52]. Chouaid et al. [53] assessed the cost-effectiveness of afatinib compared to gefitinib by use of data from the Lux-Lung 7 trial, which resulted in incremental costs of €45,211 per QALY gained. The study by NICE yielded into a cost-effectiveness ratio of £10,076 per QALY gained of afatinib versus erlotinib [52].
However, our study had several limitations. The first limitation was the use of a model-based approach (based on published RCT data), due to a lack of real-world data. Consequently, the results and conclusions of our study are dependent on the validity of the assumptions made in our model. However, various alternative assumptions were assessed through sensitivity analyses, which showed the robustness of our results.
Second, the survival probabilities of gefitinib, erlotinib, afatinib, and osimertinib were estimated by use of the EURTAC trial, which was a trial with predominantly Caucasian population. However, we also included trials with predominantly Asian population in the model, since trials with non-Asian patients for all four TKIs were not available during study period. Although Asian ethnicity is one of the risk factors for EGFR mutations [8], two studies showed no significantly different risk of progression between Asian and non-Asian patients [15, 50]. Thus, use of studies with predominantly Asian population is not expected to bias the efficacy of TKIs. Therefore, to our opinion, the results of our study could be generalised to the Dutch population.
Due to a lack of relevant data on all TKIs, we were not able to perform subgroup analyses, e.g., patients with and without brain metastases. This could be regarded as a limitation, as these analyses might give more insight into the cost-effectiveness of EGFR-TKIs in subgroups [54]. Since brain metastases occur less frequent in patients treated with osimertinib compared to patients treated with gefitinib or erlotinib, it is expected that the QALY gain for osimertinib will increase [19]. Thus, the ICER for this subgroup will be slightly lower compared to the outcomes for the total population. As the occurrence of brain metastases might have a substantial impact on the outcomes, further research on these subgroups is needed.
Furthermore, at the time of our study, the OS results of the FLAURA trial were still immature. Therefore, interim analysis of OS was used in our model. However, the use of final OS results would be more desirable, because it reduces the uncertainty of the model outcomes.
In addition, we assumed that patients treated with the first-line gefitinib, erlotinib, or afatinib all received the same second-line treatments with the same proportions, namely osimertinib (50%) or pemetrexed–cisplatin (50%) and after progression on these second-line treatments, and patients were treated with BSC. Though it may be reasonable that these proportions differ per TKI, we had no data to make such distinctions. Besides that, in reality, patients may also receive other second- or third-line treatments than those included in our model. In the ideal situation, we could fully account for the costs and effects of all second- and third-line treatments used in Dutch clinical practice. However, in the absence of any clear guidance on the second- and third-line treatment strategies after TKI failure [55, 56], we considered our assumption a valid strategy. Scenario analysis also showed a marginal impact of different second-line treatments on the costs. In further research, it is recommended to use real-world data of the first-line and second- and third-line treatment strategy, when it is available.
Furthermore, treatment costs could be overestimated somewhat as we did not adjust for dose reductions. However, adjustment for dose reductions is expected not to have a large impact on the cost-effectiveness results, since the costs related to osimertinib are high anyway. The assumption of no drug wastage is justified, because TKIs are pills and second-line pemetrexed–cisplatin was received by a relatively small proportion of patients, which is expected to have a small amount of drug wastage. The effect on the incremental differences would be negligible. However, it might be more precise when drug wastage is taken into account where relevant.
The clinical effectiveness of osimertinib for patients with EGFR-mutated NSCLC is promising, as it could improve PFS and OS. Moreover, central nervous system (CNS) progression occurred less frequent in patients treated with osimertinib compared to standard-TKI [19]. Besides the substantial clinical relevance, the costs of treating CNS metastases will also be lower for osimertinib versus standard-TKI. Despite these benefits, our results showed that osimertinib could not be regarded as cost-effective compared to all other TKIs. Therefore, it is of great importance to negotiate a lower price for osimertinib.
Conclusion
This study showed that the cost-effectiveness of afatinib compared to erlotinib is well below the Dutch threshold ratio of €80,000/QALY for treatments in this disease severity group. Osimertinib yielded a better effectiveness compared to afatinib. However, the ICER of osimertinib versus afatinib (€128,343 per QALY gained) appears to be too high given the Dutch threshold. The price of osimertinib should be reduced by 30% to become cost-effective.
References
Comprehensive Cancer Centre of the Netherlands (IKNL). Dutch cancer registration [in Dutch: Nederlandse kankerregistratie] (2016)
Sun, S., Schiller, J.H., Spinola, M., Minna, J.D.: New molecularly targeted therapies for lung cancer. J Clin Invest 117, 2740–2750 (2007)
Integraal Kankercentrum Nederland (IKNL). Non-small-cell lung cancer: Dutch guidelines [in Dutch]. 2015 (2018)
Schiller, J.H., Harrington, D., Belani, C.P., et al.: Comparison of Four Chemotherapy Regimens for Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 346, 92–98 (2002)
Novello, S., Barlesi, F., Califano, R., et al.: Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27, v1–v27 (2016)
Sleijfer, S., Verweij, J.: The price of success: cost-effectiveness of molecularly targeted agents. Clin. Pharmacol. Ther. 85, 136–138 (2009)
Lynch, T.J., Bell, D.W., Sordella, R., et al.: Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004)
Shi, Y., Au, J.S., Thongprasert, S., et al.: A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 9, 154–162 (2014)
Kerner, G.S.M.A., Schuuring, E., Sietsma, J., et al.: Common and rare EGFR and KRAS mutations in a Dutch non-small-cell lung cancer population and their clinical outcome. PLoS ONE 8, e70346 (2013)
Thunnissen, E., Smit, E.F., Nederlof, P.M., Dingemans, A.C.: EGFR-mutation in non-small cell lung cancer [in Dutch: EGFR-mutatie bij niet-kleincellig longcarcinoom]. Ned. Tijdschr. Geneeskd. 155, 1–5 (2011)
Maemondo, M., Inoue, A., Kobayashi, K., et al.: Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010)
Mitsudomi, T., Morita, S., Yatabe, Y., et al.: Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 11, 121–128 (2010)
Han, J., Park, K., Kim, S., et al.: First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J. Clin. Oncol. 30, 1122–1128 (2012)
Zhou, C., Wu, Y., Chen, G., et al.: Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12, 735–742 (2011)
Rosell, R., Carcereny, E., Gervais, R., et al.: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13, 239–246 (2012)
Sequist, L.V., Yang, J.C., Yamamoto, N., et al.: Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 31, 3327–3334 (2013)
Wu, Y., Zhou, C., Hu, C., et al.: Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 15, 213–222 (2014)
Mok, T.S., Wu, Y., Thongprasert, S., et al.: Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009)
Soria, J., Ohe, Y., Vansteenkiste, J., et al.: Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018)
Liang, W., Wu, X., Fang, W., et al.: Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations. PLoS ONE 9, e85245 (2014)
Popat, S., Mok, T., Yang, J.C., et al.: Afatinib in the treatment of EGFR mutation-positive NSCLC—a network meta-analysis. Lung Cancer 85, 230–238 (2014)
Zhang, Y., Sheng, J., Yang, Y., et al.: Optimized selection of three major EGFR-TKIs in advanced EGFR-positive non-small cell lung cancer: a network metaanalysis. Oncotarget 7, 20093–20108 (2016)
Batson, S., Mitchell, S.A., Windisch, R., Damonte, E., Munk, V.C., Reguart, N.: Tyrosine kinase inhibitor combination therapy in first-line treatment of non-small-cell lung cancer: systematic review and network meta-analysis. OncoTargets Ther. 5, 2473–2482 (2017)
Zhang, Y., Zhang, Z., Huang, X., et al.: Therapeutic efficacy comparison of 5 major EGFR-TKIs in advanced EGFR-positive non-small-cell lung cancer: a network meta-analysis based on head-to-head trials. Clin Lung Cancer 18, e333–e340 (2017)
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration (2011)
van der Linden, N., Bongers, M.L., Coupé, V.M.H., et al.: Costs of non-small cell lung cancer in the Netherlands. Lung Cancer 91, 79–88 (2016)
Dutch National Health Care Institute (ZIN). Drug costs [in Dutch: Medicijnkosten]. 2016 (2016)
Dutch National Health Care Institute (ZIN). Guidline for Economic Evaluations [in Dutch: Richtlijn Voor Het Uitvoeren Van Economische Evaluaties in De Gezondheidszorg]. Diemen: Dutch National Health Care Institute (ZIN) (2015)
Fukuoka, M., Wu, Y., Thongprasert, S., et al.: Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 29, 2866–2874 (2011)
Park, K., Tan, E., O’Byrne, K., et al.: Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 17, 577–589 (2016)
Wu, Y.L., Zhou, C., Liam, C.K., et al.: First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann. Oncol. 26, 1883–1889 (2015)
Yang, J.J., Zhou, Q., Yan, H.H., et al.: A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br. J. Cancer 116, 568–574 (2017)
Dias, S., Welton, N.J., Sutton, A.J., Ades, A.E.: A Generalized Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomized Controlled Trials, pp. 1–98. University of Sheffield, ScHARR, Sheffield (2011)
Holleman, M.S., van Tinteren, H., Groen, H.J., Al, M.J., Uyl-de Groot, C.A.: First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: a network meta-analysis. OncoTargets Ther. 12, 1413–1421 (2019)
Hoyle, M.W., Henley, W.: Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med. Res. Methodol. 11, 1–14 (2011)
Hoyle, M., Green, C., Thompson-Coon, J., et al.: Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health 13, 61–68 (2010)
Wu, B., Li, T., Cai, J., Xu, Y., Zhao, G.: Cost-effectiveness analysis of adjuvant chemotherapies in patients presenting with gastric cancer after D2 gastrectomy. BMC Cancer 14, 984 (2014)
Wang, Z., Ren, S., Li, W., Gao, G.: Frequency of the acquired resistant mutation T790 M in non-small cell lung cancer patients with active exon 19Del and exon 21 L858R: a systematic review and meta-analysis. BMC Cancer 18, 148 (2018)
Yang, J.C., Ahn, M.J., Kim, D.W., et al.: Osimertinib in pretreated T790 M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J. Clin. Oncol. 35, 1288–1296 (2017)
Soria, J., Wu, Y., Nakagawa, K., et al.: Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 16, 990–998 (2015)
Chouaid, C., Agulnik, J., Goker, E., et al.: Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol 8, 997–1003 (2013)
Harandi, A., Zaidi, A.S., Stocker, A.M., Laber, D.A.: Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J. Oncol. 2009, 567486 (2009)
Hirsh, V.: Managing treatment-related adverse events associated with egfr tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr. Oncol. 18, 126–138 (2011)
Hakkaart-van Roijen L, van der Linden N, Bouwmans CAM, Kanters T, Tan SS. Costing manual [in Dutch: Kostenhandleiding] (2016)
Dutch Healthcare Authority (NZa). DBC tariff application [in Dutch: DBC zorgproducten tariefapplicatie]. 2016 (2016)
Drummond, M.F., Sculpher, M.J., Torrance, G.W., O’Brien, B.J., Stoddart, G.L.: Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press, Oxford (2005)
Fenwick, E., Marshall, D.A., Levy, A.R., Nichol, G.: Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv. Res. 6, 52 (2006)
Aguiar Jr., P.N., Haaland, B., et al.: Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol. 4, 1080–1084 (2018)
Dutch National Health Care Institute (ZIN). Pakketadvies sluisgeneesmiddel osimertinib (Tagrisso®) bij de eerstelijnsbehandeling van patiënten met gevorderde of gemetastaseerde niet-kleincellige lonkanker (NSCLC) met activerende EGFR-mutaties. 2018
Ting, J., Tien Ho, P., Xiang, P., Sugay, A., Abdel-Sattar, M., Wilson, L.: Cost-effectiveness and value of information of erlotinib, afatinib, and cisplatin-pemetrexed for first-line treatment of advanced EGFR mutation-positive non-small-cell lung cancer in the United States. Value Health 18, 774–782 (2015)
Lee, V.W.Y., Schwander, B., Lee, V.H.F.: Effectiveness and cost-effectiveness of erlotinib versus gefitinib in first-line treatment of epidermal growth factor receptor-activating mutation-positive non-small-cell lung cancer patients in Hong Kong. Hong Kong Med. J. 20, 178–186 (2014)
National Institute for Health and Care Excellence: Afatinib for Treating Epidermal Growth Factor Receptor Mutationpositive Locally Advanced or Metastatic Non-Small-Cell Lung Cancer. National Institute for Health and Care Excellence, London (2014)
Chouaid, C., Luciani, L., LeLay, K., et al.: Cost-effectiveness analysis of afatinib versus gefitinib for first-line treatment of advanced EGFR-mutated advanced non-small cell lung cancers. J. Thorac. Oncol. 12, 1496–1502 (2017)
Wu, B., Gu, X., Zhang, Q., Xie, F.: Cost-effectiveness of osimertinib in treating newly diagnosed, advanced EGFR-mutation-positive non-small cell lung cancer. Oncologist 24, 349–357 (2019)
Kuo, C.H., Lin, S.M., Lee, K.Y., et al.: Subsequent chemotherapy improves survival outcome in advanced non-small-cell lung cancer with acquired tyrosine kinase inhibitor resistance. Clin. Lung Cancer 11, 51–56 (2010)
Wu, J.Y., Shih, J.Y., Yang, C.H., et al.: Second-line treatments after first-line gefitinib therapy in advanced nonsmall cell lung cancer. Int. J. Cancer 126, 247–255 (2010)
Lloyd, A., Nafees, B., Narewska, J., Dewilde, S., Watkins, J.: Health state utilities for metastatic breast cancer. Br. J. Cancer 95, 683–690 (2006)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Holleman, M.S., Al, M.J., Zaim, R. et al. Cost-effectiveness analysis of the first-line EGFR-TKIs in patients with non-small cell lung cancer harbouring EGFR mutations. Eur J Health Econ 21, 153–164 (2020). https://doi.org/10.1007/s10198-019-01117-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-019-01117-3