Abstract
Background
To date, there are no studies reporting an association between vitamin D and Barrett’s esophagus (BE), the precursor for esophageal adenocarcinoma (EAC).
Aims
Our aim was to study the association between serum 25-hydroxyvitamin D (25(OH)D) levels and prevalence and incidence of dysplasia/EAC in BE.
Methods
Patients from our BE Registry cohort seen between 2000 and 2012 who had serum 25(OH)D levels measured were included. Age, gender, race, BE length, hiatal hernia size, and histological findings were recorded. Patients without high-grade dysplasia (HGD)/EAC at or within 1 year of index biopsy and who had follow-up endoscopies and 25(OH)D levels were studied for incidence of dysplasia/EAC.
Results
Among 429 patients with BE, the mean 25(OH)D level was 72 ± 31.2 nmol/L. Hundred and one (23.6 %) patients had deficiency (<50 nmol/L), 149 (34.7 %) had insufficiency (50–74.9 nmol/L), and 179 (41.7 %) had normal levels of 25(OH)D. There was no association between serum 25(OH)D levels and dysplasia (p = 0.90). In the incidence cohort of 246 patients with median follow-up of 46 months, there were 34 cases of low-grade dysplasia, 12 of HGD, and 5 of EAC. Change in 25(OH)D levels did not impact progression to dysplasia/EAC (every 5 nmol/L increase from baseline, hazard ratio 0.98; p = 0.62).
Conclusions
Serum 25(OH)D levels were low in 58.3 % of our BE cohort. There was no association between 25(OH)D levels and prevalence or incidence of HGD/EAC in patients with BE. Further long-term studies are needed to study the association between vitamin D status and progression of dysplasia in BE.
Similar content being viewed by others
Introduction
Vitamin D has several anti-carcinogenic properties such as suppressing abnormal cell proliferation, promoting cell differentiation, stimulating apoptosis, and inhibiting angiogenesis. Due to its safety profile and ease of administration, it has a potential role in chemoprevention and delaying the progression of cancer. Hence, it has been studied as a chemoprotective agent in several cancers such as breast, colon, and prostate [1–4].
In contrast, the role of vitamin D in pathogenesis of esophageal adenocarcinoma (EAC) is not as well defined and may involve an immunomodulatory effect in the regulation of immune cells involved in gastro-esophageal reflux disease such as CD4 cells, macrophages and dendritic cells, and key signaling pathways including Wnt, Hedgehog, NF-kB, and IL-6–JAK–STAT as described in animal models [5]. So far, studies to show any protective association between high levels of vitamin D and esophageal cancer are equivocal [6, 7]. In fact, lower levels of vitamin D were reported to be associated with statistically decreased risk of upper gastrointestinal cancers [6]. Any protective role for vitamin D in EAC is best evaluated in a population at increased risk of developing EAC such as patients with Barrett’s esophagus (BE), the only known precursor for EAC. In addition, serum 25-hydroxyvitamin D (25(OH)D) level is considered to be the most accurate marker for assessing vitamin D status among the several methods available such as exposure to sunlight, and vitamin D and calcium intake [8]. To date, there are no reported studies that have investigated the relationship between serum 25(OH)D levels and the risk of progression of dysplasia in BE.
Our aim was to determine whether low serum 25(OH)D levels were associated with increased prevalence of dysplasia or EAC in patients with BE. We hypothesized that low serum 25(OH)D levels are associated with increased risk of dysplasia and progression toward high-grade dysplasia (HGD) or EAC. We also studied whether a change in serum 25(OH)D levels in BE patients influenced the incidence of dysplasia and EAC over time.
Methods
Study Population
Cleveland Clinic Barrett’s registry is a prospectively collected database started in 1979 of patients with a diagnosis of BE seen in the Department of Gastroenterology. The registry includes demographic data such as age, gender, race, number of endoscopies, length of Barrett’s segment, size of hiatal hernia, and histologic findings. The current study included registry patients who were aged ≥18 years and had at least one measured serum 25(OH)D level from January 1, 2000, to December 31, 2012. Patients who did not have their histological findings recorded or serum 25(OH)D levels available were excluded from the study. All patients who had at least one endoscopic exam with specialized intestinal metaplasia on esophageal biopsies and serum 25(OH)D levels measured were included in the prevalence cohort. The incidence cohort comprised of patients who had more than one surveillance endoscopy with at least 1-year follow-up. Patients who had HGD/EAC at baseline or within 1 year of index endoscopy were excluded from the incidence cohort. Follow-up was calculated from the time of first endoscopy to the last surveillance endoscopy with biopsy or until the diagnosis of HGD or EAC. The study was approved by the Cleveland Clinic Institutional Review Board on November 5, 2013.
Serum 25(OH)D Levels
Serum 25(OH)D levels were measured using DiaSorin LIAISON (25 OH) vitamin D assay in our laboratory since 2006. It is a direct competitive chemiluminescence immunoassay for quantitative determination of total 25(OH)D in serum. The antibody used in this assay reacts equally and measures both D2 and D3 forms of 25(OH)D levels. Prior to 2006, levels were measured by manual radioimmune assay. The first measured 25(OH)D value after a patient was included in the registry was considered the baseline level. Follow-up levels were recorded if available. In accordance with widely accepted cutoff values, vitamin D deficiency was defined as serum 25(OH) level <50 nmol/L, insufficiency as 50–<75 nmol/L, and normal values as >75 nmol/L for study purposes.
Biopsy Protocol and Histology
The first endoscopy with biopsies after entry into Barrett’s registry is considered the index endoscopy. As per published guidelines, a standard biopsy protocol was used for index and surveillance endoscopies, with four-quadrant biopsies every 2 cm in Barrett’s segment. In cases of known or suspected dysplasia, four-quadrant biopsies were done every 1 cm with separate biopsies for visible lesions. The histological slides were reviewed by experienced gastrointestinal pathologists. In most cases of dysplasia, the diagnosis was confirmed by either a second GI pathologist or at intra-departmental consensus conference. Dysplasia was graded based on standard Vienna classification—no dysplasia, indefinite for dysplasia (IND), low-grade dysplasia (LGD), HGD, and invasive cancer. IND and LGD were grouped together as they share similar clinical management. The primary outcomes of interest were prevalence of dysplasia and EAC and progression to HGD/EAC in BE patients in different 25(OH)D level subgroups. The secondary outcome was progression to dysplasia and EAC with changes in serum 25(OH)D levels.
Statistical Analysis
Data were presented as mean ± SD, median (interquartile range [IQR] 25th to 75th percentiles), or N (%). Serum 25(OH)D level was categorized based on common cutoff points used in previous literature into six groups: <25, 25–<37.5, 37.5–<50, 50–<75, 75–<100, and >100 nmol/L [6]. The prevalence of dysplasia was estimated as the percentage of patients with dysplasia on index endoscopy out of each 25(OH)D group. Jonckheere–Terpstra tests were used to assess differences in prevalence among different 25(OH)D groups. A time-to-event analysis was performed to assess association between 25(OH)D levels and time to progression to HGD/EAC. Kaplan–Meier plots were constructed, and log-rank tests were used to compare groups. In addition, univariable and multivariable Cox regression analyses were performed to assess the factors associated with time to progression to HGD/EAC. An automated stepwise selection method performed on 1000 bootstrap samples was used to choose the final model; baseline 25(OH)D levels were force included in the models, and variables with inclusion rates of at least 50 % were included in the final model. Multivariable analysis was not performed for progression to HGD/EAC because <20 events were observed. In addition, a subgroup analysis was performed to assess impact of changes in 25(OH)D levels with progression in subjects with available follow-up 25(OH)D levels; similar methods were employed. A p < 0.05 was considered statistically significant. SAS version 9.2 (The SAS Institute, Cary, NC) and R version 3.0.1 (The R Foundation for Statistical Computing. Vienna, Austria) were used to perform all analyses.
Results
Patient Characteristics
The prevalence cohort consisted of 429 patients with BE seen in our department from January 1, 2000, to December 31, 2012, who had serum 25(OH)D levels available (Fig. 1). They were mainly Caucasian men with mean age of 61 ± 12 years at the time of inclusion. Four hundred and twenty-three patients were on proton pump inhibitor therapy and 23 patients underwent fundoplication before or during the study period. Other patient characteristics are presented in Table 1.
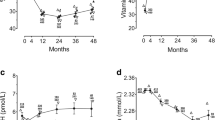
Median time between index biopsy and 25(OH)D level measurement was 33 months (IQR 8–85). There was no statistically significant difference in median serum 25(OH)D levels in different dysplasia groups (Fig. 2). There was no association between serum 25(OH)D level and the prevalence of dysplasia in BE (p = 0.90) (Table 2).
Follow-up data on surveillance endoscopies were available in 246 patients who were included in the incidence cohort (Table 3).
During a median follow-up period of 45.7 months (IQR 22.1, 90.2), progression to dysplasia occurred in 51 out of 246 patients (21 %). Of the 340 patients that had no dysplasia on index endoscopy, 34 progressed to LGD, 9 progressed to HGD, and 5 progressed to EAC. Among 52 patients with LGD on index biopsy, three patients progressed to HGD. Overall, 40 out of 429 patients had HGD/EAC at index endoscopy or within 1 year and a further 17 patients developed HGD/EAC during follow-up. Baseline 25(OH)D levels showed only marginal association for higher risk of progression to LGD/HGD/EAC on univariable Cox regression analysis [hazard ratio (HR) 1.01, 95 % CI 1.0, 1.02, p = 0.064]. After adjusting for BE length and hernia size, for every 5 nmol/L increase in baseline 25(OH)D levels, the risk of progression increased by 4 % (HR 1.04; 95 % CI 1.001, 1.08, p = 0.049). A subset of 186 patients had follow-up 25(OH)D levels available. Multivariable Cox regression model showed BE length as the only significant factor associated with progression to dysplasia/EAC (every 1 cm increase in BE length, HR 1.1, 95 % CI 1.05, 1.3, p = 0.003). Neither baseline serum 25(OH)D levels (every 5 nmol/L increase, HR 1.01, 95 % CI 0.94, 1.09, p = 0.75) nor change in serum 25(OH)D levels (every 5 nmol/L increase, HR 0.98, 95 % CI 0.93, 1.05, p = 0.62) impacted progression to dysplasia/EAC. In the time-to-event analysis, there was no difference detected in the risk of progression based on the presence of 25(OH)D deficiency (level < 50 nmol/L) (Fig. 3). Baseline serum 25(OH)D levels change in 25(OH)D levels, and follow-up 25(OH)D levels were not associated with progression to HGD/EAC (Table 4).
Discussion
In our large cohort of patients with BE, 23.6 % patients had deficient levels (<50 nmol/L), 34.7 % (149) had insufficient levels (50–74.9 nmol/L), and 41.7 % (179) had normal serum levels of 25(OH)D. We did not observe any association between 25(OH)D levels and the histological grades of dysplasia. Furthermore, the baseline 25(OH)D or change in the levels was not related to the progression to HGD/EAC on follow-up. The length of BE was the only factor associated with the progression. These findings do not support the protective effect of vitamin D in BE, a precursor for EAC.
Recently, low vitamin D level emerged as a potential risk factor for many types of cancers, especially colorectal [9], breast, and prostate cancer. Since its first description in ecologic studies [10], which showed decreased incidence of cancer among those living in southern latitudes as compared to those living in northern latitudes with inadequate sunlight, the role of vitamin D as an anti-neoplastic agent has garnered interest. The anti-neoplastic effects of vitamin D are supported by the inverse association observed between vitamin D level and risk of colorectal adenoma [11], a precursor of colorectal cancer (CRC). This may be due to mechanisms such as promoting bile acid catabolism, direct effects on cell cycle, growth factor signaling, immunomodulation, and expression of vitamin D receptor [5, 12–14]. There is further evidence from several prospective studies showing a statistically significant inverse association between serum 25(OH)D levels and CRC [9, 14, 15]. More recently, a meta-analysis of the prospective cohort studies showed decreased mortality with increased serum vitamin D levels among patients with CRC [comparing highest vs lowest 25(OH)D categories, HR 0.65, 95 % CI 0.49–0.86] [16].
However, only limited evidence exists studying the association of serum 25(OH)D levels with esophageal cancer. The studies that have examined the association between vitamin D and esophageal cancer have yielded inconsistent results with UV radiation persistently showing negative correlation and serum 25(OH)D levels showing few positive and few negative correlations [17–20]. In a population-based case–control study from Australia, patients with EAC and esophagogastric junction adenocarcinomas (EGJAC) were less likely to have high levels of cumulative lifetime UV radiation [EAC odds ratio (OR) 0.59, 95 % CI 0.35–0.99, EGJAC OR 0.55, 95 % CI 0.34–0.90] compared with population controls [7]. The associations were independent of age, sex, body mass index, reflux, smoking, alcohol consumption, and H. pylori status. Giovannucci et al. [21] reported a statistically significant inverse correlation between predicted serum 25(OH)D levels and incidence of esophageal cancer in the Health Professionals Follow-up cohort. Each 25-nmol/L increase in predicted 25(OH)D levels corresponded to a 63 % decrease in esophageal cancer incidence. Finally, a North American study reported a 27 % increase in incidence and 36 % increase in mortality of esophageal cancer in populations receiving an annual average of 650 kJ/m2 year UV exposure versus 1540 kJ/m2 year in Caucasian men [22].
In contrast, a few studies reported a higher risk of esophageal cancer with higher vitamin D levels. One of the major studies was a case–control study by Mulholland et al. [6] exploring the association between patient’s self-reported intake of vitamin D, and BE and EAC. The study reported no association between vitamin D and BE, but a direct association between vitamin D intake and EAC; this persisted after adjusting for confounders (OR highest tertile vs lowest tertile of vitamin D intake, 1.99, 95 % CI 1.03–3.86, p = 0.02), but did not persist after adjusting for normal weight individuals, individuals negative for H. pylori, and never or nonsmokers. Two other studies, which were conducted in China, showed direct association of vitamin D with esophageal squamous cell cancer (ESCC) and esophageal squamous dysplasia, a precancerous lesion of ESCC, but did not report any association with EACs [20, 23]. In addition, a large case–control study that combined eight prospective cohorts with 256 cases of esophageal cancer (142 ESCC and 104 EAC) showed no statistically significant association [24]. But on subgroup analysis, nonsmokers and Asians with lowest serum 25(OH)D levels had lower risk of upper GI cancers (OR 0.53 and 0.55, respectively). This discrepancy between the studies may be due to the study design involving different populations and varying methods of assessing vitamin D status such as dietary intake, UV radiation, and serum 25(OH)D levels.
The lack of association observed in our study may be due to two factors: (1) Serum 25(OH)D levels may not accurately reflect the intracellular effect of 1,25-hydroxyvitamin D which has the active biological role in modulating neoplastic pathways and (2) a type II error failing to detect a true association from either a limited sample size or a lack of sufficiently long follow-up period. It has been hypothesized that long-term vitamin D status is more relevant to carcinogenesis than current or recent vitamin D status [7]. Of note, a clinical trial is underway to examine the effect of vitamin D supplementation in BE with dysplasia [25]. This may help elucidate our understanding of the association between vitamin D and BE.
To our knowledge, there are no epidemiologic studies that have tested the association between serum 25(OH)D levels and BE. One of the major strengths of our study is the use of serum 25(OH)D levels which is the most stable metabolite of vitamin D with a half-life of about 3 weeks, making it the most suitable indicator of vitamin D status instead of indirect measures such as exposure to UV radiation or nutritional intake of vitamin D. Furthermore, serum 25(OH)D levels were measured in a single laboratory using DiaSorin LIAISON assay which limits variation due the disparity between different laboratories and techniques of measurement. In addition, a subgroup of these patients had several surveillance endoscopies and follow-up serum 25(OH) levels which allowed us to study progression to dysplasia/EAC. Also, our study population consisted of mostly Caucasian men which mirrors the community prevalence of at-risk population for BE. Most of the slides have been reviewed by expert gastrointestinal pathologists to confirm the presence of dysplasia as per recommendations by national guidelines.
As this is an observational study, the time interval between index endoscopy and serum 25(OH)D level measurement was not standardized and seasonal variations in levels were not taken into account. The median time between index biopsy and 25(OH)D level measurement was 33 months (IQR 8–85) which could have impacted our findings. Also, it is not known whether a single measurement of serum 25(OH)D is an accurate indicator of a person’s long-term vitamin D status. Supporting evidence comes from a recent study evaluating intra-individual variability in serum 25(OH)D concentrations over a 5-year period which showed stable levels [26]. Although we measured serum 25(OH)D levels, a wide variety of other factors which influence vitamin D status such as dietary and supplemental intakes of vitamin D and calcium, long-term sunlight exposure, alcohol intake, and the season of measurement of vitamin D were not studied. However, serum 25(OH)D levels are considered to be the single best biomarker for vitamin D status of body and therefore used in this study. Furthermore, there is no consensus regarding the definition of vitamin D deficiency or insufficiency which may limit generalizability of results. To address this variation, we used cutoff values based on cohort consortium vitamin D pooling project studies [6, 20, 24].
In conclusion, we did not observe any association between serum 25(OH)D levels or change in levels with increased prevalence or incidence of HGD/EAC in patients with BE. Future prospective studies are needed to assess not only serum 25(OH)D levels but also other markers of vitamin D status such as dietary intake and supplementation and ultraviolet radiation exposure over a prolonged time frame as any impact of vitamin D on esophageal carcinogenesis may have a long latency period.
References
Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nature reviews. Cancer. 2007;7:684–700.
Diaz GD, Paraskeva C, Thomas MG, et al. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60:2304–2312.
Holt PR, Arber N, Halmos B, et al. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomark Prev. 2002;11:113–119.
Holt PR, Bresalier RS, Ma CK, et al. Calcium plus vitamin D alters preneoplastic features of colorectal adenomas and rectal mucosa. Cancer. 2006;106:287–296.
Trowbridge R, Kizer RT, Mittal SK, et al. 1,25-dihydroxyvitamin D in the pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Expert Rev Clin Immunol. 2013;9:517–533.
Mulholland HG, Murray LJ, Anderson LA, et al. Vitamin D, calcium and dairy intake, and risk of oesophageal adenocarcinoma and its precursor conditions. Br J Nutr. 2011;106:732–741.
Tran B, Lucas R, Kimlin M, et al. Association between ambient ultraviolet radiation and risk of esophageal cancer. Am J Gastroenterol. 2012;107:1803–1813.
Food and Nutrition Board of the Institute of Medicine. Vitamin D. In: Young VR, Garza C, Atkinson SA, Munro IC, eds. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, Fluoride. Washington, DC: National Academies Press; 1997:250.
Chung M, Lee J, Terasawa T, et al. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:827–838.
Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–231.
Fedirko V, Bostick RM, Goodman M, Flanders WD, Gross MD. Blood 25-hydroxyvitamin D3 concentrations and incident sporadic colorectal adenoma risk: a pooled case-control study. Am J Epidemiol. 2010;172:489–500.
Ebert R, Schutze N, Adamski J, et al. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol. 2006;248:149–159.
Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nature reviews. Cancer. 2003;3:601–614.
Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–3782.
Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res. 2011;4:735–743.
Maalmi H, Ordonez-Mena JM, Schottker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer. 2014;50:1510–1521.
La Vecchia C, Ferraroni M, D’Avanzo B, et al. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol Biomark Prev. 1994;3:393–398.
Pelucchi C, Tramacere I, Bertuccio P, et al. Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study. Ann Oncol. 2009;20:160–165.
Chen W, Dawsey SM, Qiao YL, et al. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97:123–128.
Abnet CC, Chen W, Dawsey SM, et al. Serum 25(OH)-vitamin D concentration and risk of esophageal squamous dysplasia. Cancer Epidemiol Biomark Prev. 2007;16:1889–1893.
Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459.
Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer. 2006;6:264.
Chen W, Clements M, Rahman B, Zhang S, Qiao Y, Armstrong BK. Relationship between cancer mortality/incidence and ambient ultraviolet B irradiance in China. Cancer Causes Control. 2010;21:1701–1709.
Abnet CC, Chen Y, Chow WH, et al. Circulating 25-hydroxyvitamin D and risk of esophageal and gastric cancer: cohort consortium vitamin D pooling project of rarer cancers. Am J Epidemiol. 2010;172:94–106.
Cummings LC, Willis J, Cooper GS, et al. Effects of vitamin D supplementation on Barrett’s esophagus. Gastroenterology. 2013;144:s-696.
Hofmann JN, Yu K, Horst RL, et al. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomark Prev. 2010;19:927–931.
Author Contributions
Prashanthi N. Thota MD, FACG: Study design, data collection, data analysis and interpretation, and drafting the manuscript. Gaurav Kistangari MD, MPH: Data analysis and interpretation, and drafting the manuscript. Prabhdeep Singh, MD: Data collection and reviewing the manuscript. Linda Cummings, MD: Study design, data analysis and interpretation, and drafting the manuscript. Kaveh Hajifathalian, MD: Data Collection and reviewing the manuscript. Rocio Lopez MS: Study design, data analysis and interpretation, and reviewing the manuscript. Madhusudhan R. Sanaka MD, FACG: Study design, data analysis and interpretation, and reviewing the manuscript. The final draft submitted has been approved by all authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Guarantor of the Article
Prashanthi N. Thota, MD, FACG.
Rights and permissions
About this article
Cite this article
Thota, P.N., Kistangari, G., Singh, P. et al. Serum 25-Hydroxyvitamin D Levels and the Risk of Dysplasia and Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus. Dig Dis Sci 61, 247–254 (2016). https://doi.org/10.1007/s10620-015-3823-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3823-5