Summary
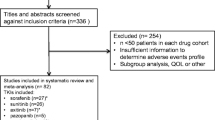
Axitinib is a potent, selective vascular endothelial growth factor receptor (VEGFR) inhibitor. We have performed a systematic analysis to investigate the risk of hand-foot skin reaction (HFSR) to axitinib and compare the differences in incidences between sorafenib, sunitinib, pazopanib and axitinib. Relevant studies were identified from PubMed (1998–2012). Eligible studies were limited to prospective Phase II-III clinical trials in which cancer patients were treated with axitinib monotherapy at a starting dose of 5 mg orally twice daily. Incidence, relative risk (RR), and 95 % confidence intervals were calculated using random-effects or fixed-effects models based on heterogeneity of included studies. A total of 984 patients from 6 prospective clinical trials were included in the analysis. The overall incidence of all-grade and high-grade HFSR was 29.2 % (95 % CI: 14.0–51.1 %) and 9.6 % (95 % CI: 4.2–20.7 %), respectively. The relative risks of all-grade and high-grade HFSR to axitinib compared to sorafenib were decreased for all-grade (RR = 0.54, 95 % CI: 0.44–0.65, p < 0.001) and high-grade HFSR (RR = 0.31, 95 % CI: 0.19–0.52, p < 0.001). The risk of all-grade and high-grade HFSR to axitinib, sunitinib and sorafenib was significantly higher as compared to pazopanib (RR = 6.49, 95 % CI: 4.65–9.05, p < 0.001; RR = 6.40, 95 % CI: 3.60–11.37, p < 0.001, and RR = 4.20, 95 % CI: 3.07–5.75, p < 0.001; RR = 3.67, 95 % CI: 2.15–6.24, p < 0.001, and RR = 7.51, 95 % CI: 5.5–10.3, p < 0.001; RR = 5.93, 95 % CI: 3.5–10.0, p < 0.001, respectively). Similar to sorafenib and sunitinib, axitinib is associated with a significant risk of HFSR, despite having an increased specificity for VEGF receptors. These findings underscore the importance of supportive dermatologic care in patients treated with axitinib, in order to maintain quality of life, adherence, and persistence to therapy.




Similar content being viewed by others
References
Igarashi H, Esumi M, Ishida H, Okada K (2002) Vascular endothelial growth factor overexpression is correlated with von Hippel-Lindau tumor suppressor gene inactivation in patients with sporadic renal cell carcinoma. Cancer 95(1):47–53. doi:10.1002/cncr.10635
Sawhney R, Kabbinavar F (2008) Angiogenesis and angiogenic inhibitors in renal cell carcinoma. Curr Urol Rep 9(1):26–33
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol: Off J Am Soc Clin Oncol 27(22):3584–3590. doi:10.1200/JCO.2008.20.1293
Motzer RJ, Michaelson MD, Rosenberg J, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Wilding G (2007) Sunitinib efficacy against advanced renal cell carcinoma. J Urol 178(5):1883–1887. doi:10.1016/j.juro.2007.07.030
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134. doi:10.1056/NEJMoa060655
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol: Off J Am Soc Clin Oncol 28(6):1061–1068. doi:10.1200/JCO.2009.23.9764
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N, Investigators AT (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370(9605):2103–2111. doi:10.1016/S0140-6736(07)61904-7
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol: Off J Am Soc Clin Oncol 26(33):5422–5428. doi:10.1200/JCO.2008.16.9847
Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O’Connor P, Shalinsky DR, Bender SL (2008) Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res: Off J Am Assoc Cancer Res 14(22):7272–7283. doi:10.1158/1078-0432.CCR-08-0652
U.S. Food and Drug Administration (2011) FDA Briefing Document, Oncologic Drugs Advisory Committee Meeting, NDA 20324 Axitinib (Inlyta). http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/oncologicdrugsadvisorycommittee/ucm282290.pdf. Accessed 12 Nov 2012
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378(9807):1931–1939. doi:10.1016/S0140-6736(11)61613-9
Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, Letourneau R, Bajetta E, Pithavala Y, Bycott P, Trask P, Liau K, Ricart AD, Kim S, Rixe O (2008) Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet 371(9630):2101–2108. doi:10.1016/S0140-6736(08)60661-3
Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E (2011) Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. The Lancet Oncol 12(3):256–262. doi:10.1016/S1470-2045(11)70004-3
Fruehauf J, Lutzky J, McDermott D, Brown CK, Meric JB, Rosbrook B, Shalinsky DR, Liau KF, Niethammer AG, Kim S, Rixe O (2011) Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res: Off J Am Assoc Cancer Res 17(23):7462–7469. doi:10.1158/1078-0432.CCR-11-0534
Schiller JH, Larson T, Ou SH, Limentani S, Sandler A, Vokes E, Kim S, Liau K, Bycott P, Olszanski AJ, von Pawel J (2009) Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol: Off J Am Soc Clin Oncol 27(23):3836–3841. doi:10.1200/JCO.2008.20.8355
Rini BI, Grünwald V, Fishman MN, Melichar B, Ueda T, Karlov PA, Bair AH, Chen Y, Kim S, Jonasch E, Cleveland Clinic Taussig Cancer Institute C, OH, Hannover Medical School H, Germany, H. Lee Moffitt Cancer Center & Research Institute T, FL, University Hospital O, Czech Republic, Division of Urology CCC, Chiba, Japan, City Clinical Oncology Dispensary SP, Russia, Pfizer Oncology SD, CA, University of Texas M. D. Anderson Cancer Center H, TX (2012) Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol 30, 2012 (suppl; abstr 4503)
ClinicalTrials.gov. Available at http://www.clinicaltrials.gov. Accessed 10 Nov 2012
Nardone B, Hensley JR, Kulik L, West DP, Mulcahy M, Rademaker A, Lacouture ME (2012) The effect of hand-foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. J Drugs Dermatol: JDD 11(11):e61–e65
Sibaud V, Dalenc F, Chevreau C, Roche H, Delord JP, Mourey L, Lacaze JL, Rahhali N, Taieb C (2011) HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist 16(10):1469–1478. doi:10.1634/theoncologist.2011-0033
Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP (2008) Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist 13(9):1001–1011. doi:10.1634/theoncologist.2008-0131
Balagula Y, Lacouture ME, Cotliar JA (2010) Dermatologic toxicities of targeted anticancer therapies. J Support Oncol 8(4):149–161
Lacouture ME, Reilly LM, Gerami P, Guitart J (2008) Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 19(11):1955–1961. doi:10.1093/annonc/mdn389
Sonpavde G, Hutson TE, Rini BI (2008) Axitinib for renal cell carcinoma. Expert Opin Investig Drugs 17(5):741–748. doi:10.1517/13543784.17.5.741
Chu D, Lacouture ME, Fillos T, Wu S (2008) Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol 47(2):176–186. doi:10.1080/02841860701765675
Chu D, Lacouture ME, Weiner E, Wu S (2009) Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer 7(1):11–19. doi:10.3816/CGC.2009.n.002
Balagula Y, Wu S, Su X, Feldman DR, Lacouture ME (2012) The risk of hand foot skin reaction to pazopanib, a novel multikinase inhibitor: a systematic review of literature and meta-analysis. Investig New Drugs 30(4):1773–1781. doi:10.1007/s10637-011-9652-2
Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB (2008) Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol: Off J Am Soc Clin Oncol 26(29):4708–4713. doi:10.1200/JCO.2007.15.9566
Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, Rosbrook B, Trask PC, Wood L, Dutcher JP (2009) Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol: Off J Am Soc Clin Oncol 27(27):4462–4468. doi:10.1200/JCO.2008.21.7034
Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, Bolte O, Motzer RJ, Bycott P, Liau KF, Freddo J, Trask PC, Kim S, Rini BI (2007) Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. The Lancet Oncol 8(11):975–984. doi:10.1016/S1470-2045(07)70285-1
Tomita Y, Uemura H, Fujimoto H, Kanayama HO, Shinohara N, Nakazawa H, Imai K, Umeyama Y, Ozono S, Naito S, Akaza H (2011) Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell Carcinoma. Eur J Cancer 47(17):2592–2602. doi:10.1016/j.ejca.2011.07.014
Inlyta® (axitinib) tablets [full prescribing information]. New York: Pfizer, 2012.
van Geel RM, Beijnen JH, Schellens JH (2012) Concise drug review: pazopanib and axitinib. Oncologist 17(8):1081–1089. doi:10.1634/theoncologist.2012-0055
U. S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute (9 Aug 2006) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Accessed 5 Nov 2012.
Porta C, Paglino C, Imarisio I, Bonomi L (2007) Uncovering Pandora’s vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin exp Med 7(4):127–134. doi:10.1007/s10238-007-0145-8
Autier J, Escudier B, Wechsler J, Spatz A, Robert C (2008) Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol 144(7):886–892. doi:10.1001/archderm.144.7.886
Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, Kuo TT, Hsieh JJ, Chang JW (2008) Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
Huggins RH, KuzelTM, Anderson RT, West DP, Lacouture ME (2008) Hand foot skin reaction (HFSR) by the multikinase inhibitors (MKIs) sorafenib and sunitinib: Impact on quality of life (QoL). J Clin Oncol 26: 2008 (suppl; abstr 16122)
Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC, Kong HH (2009) Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res: Off J Am Assoc Cancer Res 15(4):1411–1416. doi:10.1158/1078-0432.CCR-08-1141
Lacouture ME, Boerner SA, Lorusso PM (2006) Non-rash skin toxicities associated with novel targeted therapies. Clin Lung Cancer 8(Suppl 1):S36–S42
Jain L, Sissung TM, Danesi R, Kohn EC, Dahut WL, Kummar S, Venzon D, Liewehr D, English BC, Baum CE, Yarchoan R, Giaccone G, Venitz J, Price DK, Figg WD (2010) Hypertension and hand-foot skin reactions related to VEGFR2 genotype and improved clinical outcome following bevacizumab and sorafenib. J Exp Clin Cancer Res: CR 29:95. doi:10.1186/1756-9966-29-95
Gomez P, Lacouture ME (2011) Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: experience in breast cancer. Oncologist 16(11):1508–1519. doi:10.1634/theoncologist.2011-0115
Beldner M, Jacobson M, Burges GE, Dewaay D, Maize JC Jr, Chaudhary UB (2007) Localized palmar-plantar epidermal hyperplasia: a previously undefined dermatologic toxicity to sorafenib. Oncologist 12(10):1178–1182. doi:10.1634/theoncologist.12-10-1178
Strumberg D, Awada A, Hirte H, Clark JW, Seeber S, Piccart P, Hofstra E, Voliotis D, Christensen O, Brueckner A, Schwartz B (2006) Pooled safety analysis of BAY 43–9006 (sorafenib) monotherapy in patients with advanced solid tumours: is rash associated with treatment outcome? Eur J Cancer 42(4):548–556. doi:10.1016/j.ejca.2005.11.014
Jacobi U, Waibler E, Schulze P, Sehouli J, Oskay-Ozcelik G, Schmook T, Sterry W, Lademann J (2005) Release of doxorubicin in sweat: first step to induce the palmar-plantar erythrodysesthesia syndrome? Ann Oncol: Off J Eur Soc Med Oncol/ESMO 16(7):1210–1211. doi:10.1093/annonc/mdi204
Jain L, Gardner ER, Figg WD, Chernick MS, Kong HH (2010) Lack of association between excretion of sorafenib in sweat and hand-foot skin reaction. Pharmacotherapy 30(1):52–56. doi:10.1592/phco.30.1.52
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol: Off J Am Soc Clin Oncol 24(1):25–35. doi:10.1200/JCO.2005.02.2194
Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 66(24):11851–11858. doi:10.1158/0008-5472.CAN-06-1377
Rosen A, Balagula Y, Goldfarb S, Lacouture ME (2012) Dermatologic Adverse Events during Treatment. In: Berger AM, Shuster JL, Von Roenn JH (eds) Principles and Practice of Palliative Care and Supportive Oncology, 4e, Philadelphia. Wolters Kluwer Health / Lippincott Williams & Wilkins, pp 333–347
Breccia M, Carmosino I, Russo E, Morano SG, Latagliata R, Alimena G (2005) Early and tardive skin adverse events in chronic myeloid leukaemia patients treated with imatinib. Eur J Haematol 74(2):121–123. doi:10.1111/j.1600-0609.2004.00351.x
Schenone S, Bondavalli F, Botta M (2007) Antiangiogenic agents: an update on small molecule VEGFR inhibitors. Curr Med Chem 14(23):2495–2516
Rosen AC, Wu S, Damse A, Sherman E, Lacouture ME (2012) Risk of rash in cancer patients treated with vandetanib: systematic review and meta-analysis. J Clin Endocrinol Metab 97(4):1125–1133. doi:10.1210/jc.2011-2677
Launay-Vacher V, Deray G (2009) Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anti-Cancer Drugs 20(1):81–82. doi:10.1097/CAD.0b013e3283161012
Lim WT, Ng QS, Ivy P, Leong SS, Singh O, Chowbay B, Gao F, Thng CH, Goh BC, Tan DS, Koh TS, Toh CK, Tan EH (2011) A Phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res: Off J Am Assoc Cancer Res 17(16):5481–5489. doi:10.1158/1078-0432.CCR-10-3409
Yoo C, Kim JE, Lee JL, Ahn JH, Lee DH, Lee JS, Na S, Kim CS, Hong JH, Hong B, Song C, Ahn H (2010) The efficacy and safety of sunitinib in Korean patients with advanced renal cell carcinoma: high incidence of toxicity leads to frequent dose reduction. Jpn J Clin Oncol 40(10):980–985. doi:10.1093/jjco/hyq073
Zhang H, Dong B, Lu JJ, Yao X, Zhang S, Dai B, Shen Y, Zhu Y, Ye D, Huang Y (2009) Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter study. BMC Cancer 9:249. doi:10.1186/1471-2407-9-249
Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T (2005) Cutaneous side-effects of kinase inhibitors and blocking antibodies. The Lancet Oncol 6(7):491–500. doi:10.1016/S1470-2045(05)70243-6
Dranitsaris G, Vincent MD, Yu J, Huang L, Fang F, Lacouture ME (2012) Development and validation of a prediction index for hand-foot skin reaction in cancer patients receiving sorafenib. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 23(8):2103–2108. doi:10.1093/annonc/mdr580
Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME (2009) Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist 14(3):291–302. doi:10.1634/theoncologist.2008-0237
Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, Song T, Zhou W, Wang H, Yang W, Wang X, Yang Y, Shi L, Bai Y, Ye S, Zhongshan Hospital FU, Shanghai, China, The Third Affiliated Hospital of Sun Yat-sen University G, China, 301 Military Hospital B, China; Liver Transplantation Center of the 3rd Affiliated Hospital of Sun Yat-sen University, Guangzhou, China, Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University S, China, Guangdong Provincial People’s Hospital G, China, Tianjin Cancer Hospital T, China; Eastern Hepatobiliary Hospital of the Second Military Medical University, Shanghai, China, Jilin Provincial Tumor Hospital J, China, Union Hospital of Fujian Medical University F, China, The 81 Hospital of the Chinese People’s Liberation Army N, China, 302 Military Hospital of China B, China, Heilongjiang Provincial Cancer Hospital H, China (2012) A randomized controlled phase II study of the prophylactic effect of urea-based cream on the hand-foot skin reaction associated with sorafenib in advanced hepatocellular carcinoma. J Clin Oncol 30, 2012 (suppl; abstr 4008)
Trask PC, Bushmakin AG, Cappelleri JC, Bycott P, Liau K, Kim S (2008) Health-related quality of life during treatment for renal cell carcinoma: results from a phase II study of axitinib. Acta Oncol 47(5):843–851. doi:10.1080/02841860802047395
U. S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute (2010) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed 5 Nov 2012
Acknowledgments
M.E.L. is supported by a Career Development Award from the Dermatology Foundation. We thank Dr. Allan C. Halpern for his review of this manuscript.
Source of support
Memorial Sloan-Kettering; Dermatology Foundation Career and Development Award
Disclosure statement
AF, SW and AH have nothing to disclose.
MEL is supported by a Career Development Award from the Dermatology Foundation. He has a consultant role with AstraZeneca Pharmaceuticals. He is also receiving research funding from Berg, Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, A., Wu, S., Ho, A.L. et al. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs 31, 787–797 (2013). https://doi.org/10.1007/s10637-013-9927-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9927-x