Abstract
Purpose
To evaluate the safety of gadobutrol for magnetic resonance imaging in a prospective, non-interventional, post-marketing surveillance in Japan.
Materials and methods
Gadobutrol was administered in accordance with Japanese prescribing information over a 2-year enrollment period, using a standardized questionnaire to collect information. The primary outcome was the incidence of adverse reactions (ARs) following gadobutrol injection.
Results
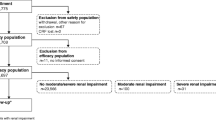
Questionnaire data were analyzed for 3337 patients (age, 58.1 ± 17.4 years [mean±SD]). Gadobutrol was administered at a dose of 0.10 ± 0.02 mL/kg body weight. Thirty-three patients were observed to have 42 ARs suspected to be due to gadobutrol, an incidence proportion of 0.99%; 29 ARs were acute (<1 h post-injection)—including one case of severe acute AR (0.03%). Patient subpopulations (with hepatic, renal, cardiovascular diseases) did not differ markedly in AR proportions categorized by age, sex, presence of comorbidity, or imaging indication. No cases of nephrogenic systemic fibrosis were reported. Investigators rated images as improved or profoundly improved following gadobutrol injection in 91.1% of examinations.
Conclusion
Gadobutrol was well tolerated with a good safety profile in this post-marketing surveillance of a large patient population in Japan.




Similar content being viewed by others
References
Huppertz A, Rohrer M. Gadobutrol, a highly concentrated MR-imaging contrast agent: its physicochemical characteristics and the basis for its use in contrast-enhanced MR angiography and perfusion imaging. Eur Radiol. 2004;14(Suppl 5):M12–8.
Tombach B, Benner T, Reimer P, Schuierer G, Fallenberg EM, Geens V, et al. Do highly concentrated gadolinium chelates improve MR brain perfusion imaging? Intraindividually controlled randomized crossover concentration comparison study of 0.5 versus 1.0 mol/L gadobutrol. Radiology. 2003;226:880–8.
Tombach B, Heindel W. Value of 1.0-M gadolinium chelates: review of preclinical and clinical data on gadobutrol. Eur Radiol. 2002;12:1550–6.
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–24.
Shen Y, Goerner FL, Snyder C, Morelli JN, Hao D, Hu D, et al. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015;50:330–8.
Endrikat J, Vogtlaender K, Dohanish S, Balzer T, Breuer J. Safety of gadobutrol: results from 42 clinical phase II to IV studies and postmarketing surveillance after 29 million applications. Invest Radiol. 2016;51:537–43.
Forsting M, Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol—a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur J Radiol. 2010;74:e186–92.
Hahn G, Sorge I, Gruhn B, Glutig K, Hirsch W, Bhargava R, et al. Pharmacokinetics and safety of gadobutrol-enhanced magnetic resonance imaging in pediatric patients. Invest Radiol. 2009;44:776–83.
Kunze C, Mentzel HJ, Krishnamurthy R, Fleck R, Stenzel M, Bhargava R, et al. Pharmacokinetics and safety of macrocyclic gadobutrol in children aged younger than 2 years including term newborns in comparison to older populations. Invest Radiol. 2016;51:50–7.
Prince MR, Lee HG, Lee CH, Youn SW, Lee IH, Yoon W, et al. Safety of gadobutrol in over 23,000 patients: the GARDIAN study, a global multicentre, prospective, non-interventional study. Eur Radiol. 2017;27:286–95.
Glutig K, Bhargava R, Hahn G, Hirsch W, Kunze C, Mentzel HJ, et al. Safety of gadobutrol in more than 1,000 pediatric patients: subanalysis of the GARDIAN study, a global multicenter prospective non-interventional study. Pediatr Radiol. 2016;46:1317–23.
Voth M, Rosenberg M, Breuer J. Safety of gadobutrol, a new generation of contrast agents: experience from clinical trials and postmarketing surveillance. Invest Radiol. 2011;46:663–71.
Malik M, Hnatkova K, Schmidt A, Smetana P. Correction for QT/RR hysteresis in the assessment of drug-induced QTc changes—cardiac safety of gadobutrol. Ann Noninvasive Electrocardiol. 2009;14:242–50.
Balzer JO, Loewe C, Davis K, Goyen M, Leiner T, Meaney JF, et al. Safety of contrast-enhanced MR angiography employing gadobutrol 1.0 M as contrast material. Eur Radiol. 2003;13:2067–74.
Tombach B, Bremer C, Reimer P, Kisters K, Schaefer RM, Geens V, et al. Renal tolerance of a neutral gadolinium chelate (gadobutrol) in patients with chronic renal failure: results of a randomized study. Radiology. 2001;218:651–7.
Frenzel T, Lengsfeld P, Schirmer H, Hutter J, Weinmann HJ. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43:817–28.
Sieber MA, Lengsfeld P, Frenzel T, Golfier S, Schmitt-Willich H, Siegmund F, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18:2164–73.
Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–8.
Thomsen HS, Morcos SK, Almen T, Bellin MF, Bertolotto M, Bongartz G, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23:307–18.
American College of Rheumatology. ACR Manual on Contrast Media. Version. 10.3. 2007. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf.
European Medicines Agency. EMA./425304/2010. Rev.1. Patient Health Protection. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2010/07/WC500094268.pdf.
Kanda T, Oba H, Toyoda K, Kitajima K, Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol. 2016;34:3–9.
Cao Y, Huang DQ, Shih G, Prince MR. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol. 2016;206:414–9.
Radbruch A, Haase R, Kieslich PJ, Weberling LD, Kickingereder P, Wick W, et al. No signal intensity increase in the dentate nucleus on unenhanced T1-weighted MR images after more than 20 serial injections of macrocyclic gadolinium-based contrast agents. Radiology. 2017;282:699–707.
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol. 2015;50:805–10.
Renz DM, Kumpel S, Bottcher J, Pfeil A, Streitparth F, Waginger M, et al. Comparison of unenhanced T1-weighted signal intensities within the dentate nucleus and the globus pallidus after serial applications of gadopentetate dimeglumine versus gadobutrol in a pediatric population. Invest Radiol. 2018;53:119–27.
Schlemm L, Chien C, Bellmann-Strobl J, Dorr J, Wuerfel J, Brandt AU, et al. Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler. 2017;23:963–72.
Fingerhut S, Niehoff AC, Sperling M, Jeibmann A, Paulus W, Niederstadt T, et al. Spatially resolved quantification of gadolinium deposited in the brain of a patient treated with gadolinium-based contrast agents. J Trace Elem Med Biol. 2018;45:125–30.
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276:228–32.
Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51:447–53.
Roberts DR, Welsh CA, LeBel DP 2nd, Davis WC. Distribution map of gadolinium deposition within the cerebellum following GBCA administration. Neurology. 2017;88:1206–8.
Michaely HJ, Aschauer M, Deutschmann H, Bongartz G, Gutberlet M, Woitek R, et al. Gadobutrol in renally impaired patients: results of the GRIP study. Invest Radiol. 2017;52:55–60.
European Society of Urogenital Radiology. ESUR guidelines on contrast media, Version 9.0. 2017. http://www.esur.org/guidelines/.
Tsushima Y, Ishiguchi T, Murakami T, Hayashi H, Hayakawa K, Fukuda K, et al. Safe use of iodinated and gadolinium-based contrast media in current practice in Japan: a questionnaire survey. Jpn J Radiol. 2016;34:130–9.
Imai E, Yasuda Y, Makino H. Japan Association of Chronic Kidney Disease Initiatives (J-CKDI). JMAJ. 2011;54:406–8.
Michel A, Abudulah A, Schumann W. Spontaneous reporting of adverse drug reactions: estimation of underreporting for contrast media reactions in 14 countries. Pharmacoepidemiol Drug Saf. 2001;10:34.
Weisbord SD, Mor MK, Resnick AL, Hartwig KC, Palevsky PM, Fine MJ. Incidence and outcomes of contrast-induced AKI following computed tomography. Clin J Am Soc Nephrol. 2008;3:1274–81.
Soyer P, Dohan A, Patkar D, Gottschalk A. Observational study on the safety profile of gadoterate meglumine in 35,499 patients: The SECURE study. J Magn Reson Imaging. 2017;45:988–97.
de Kerviler E, Maravilla K, Meder JF, Naggara O, Dubourdieu C, Jullien V, et al. Adverse reactions to gadoterate meglumine: review of over 25 years of clinical use and more than 50 million doses. Invest Radiol. 2016;51:544–51.
Fakhran S, Alhilali L, Kale H, Kanal E. Assessment of rates of acute adverse reactions to gadobenate dimeglumine: review of more than 130,000 administrations in 7.5 years. AJR Am J Roentgenol. 2015;204:703–6.
Abujudeh HH, Kosaraju VK, Kaewlai R. Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: experience with 32,659 injections. AJR Am J Roentgenol. 2010;194:430–4.
Shellock FG, Parker JR, Venetianer C, Pirovano G, Spinazzi A. Safety of gadobenate dimeglumine (MultiHance): summary of findings from clinical studies and postmarketing surveillance. Invest Radiol. 2006;41:500–9.
Matsumura T, Hayakawa M, Shimada F, Yabuki M, Dohanish S, Palkowitsch P, et al. Safety of gadopentetate dimeglumine after 120 million administrations over 25 years of clinical use. Magn Reson Med Sci. 2013;12:297–304.
Ishiguchi T, Takahashi S. Safety of gadoterate meglumine (Gd-DOTA) as a contrast agent for magnetic resonance imaging: results of a post-marketing surveillance study in Japan. Drugs R D. 2010;10:133–45.
Japan Radiological Society. Japanese College of Radiology. The Japanese Imaging Guideline. 2013. http://www.radiology.jp/content/files/diagnostic_imaging_guidelines_2013_e.pdf.
Noda Y, Goshima S, Namimoto T, Shinkawa N, Nakagawa M, Kajita K, et al. Simultaneous acquisition of MR angiography and diagnostic images of abdomen at view-sharing multiarterial phases and comparing the effect of two different contrast agents. J Magn Reson Imaging. 2018;48:102–10.
Acknowledgements
This surveillance was funded by Bayer Yakuhin, Ltd. Editorial assistance was provided by Bill Wolvey of PAREXEL, which was funded by Bayer Yakuhin, Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YT has no conflict of interest. KA received lecture fees and honoraria for chairman from Bayer Yakuhin, Ltd. GS is an employee of Bayer Yakuhin, Ltd. HM is an employee of Bayer Yakuhin, Ltd. MC is an employee of Bayer Yakuhin, Ltd. TS is an employee of Bayer Yakuhin, Ltd. JE is an employee of Bayer AG.
Ethical standards and informed consent
This surveillance was conducted in compliance with the GPSP and GVP of the Ministry of Health, Labor and Welfare in Japan. Approval by the ethics committee of each institution was not mandatory, since GPSP and GVP do not require such approval for a PMS. Informed consent from each patient was waived
About this article
Cite this article
Tsushima, Y., Awai, K., Shinoda, G. et al. Post-marketing surveillance of gadobutrol for contrast-enhanced magnetic resonance imaging in Japan. Jpn J Radiol 36, 676–685 (2018). https://doi.org/10.1007/s11604-018-0778-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0778-4