Abstract
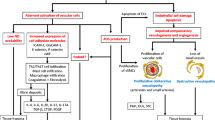
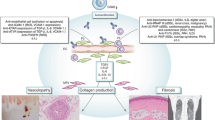
Described as an autoimmune collagen vascular disease, the most striking feature of scleroderma may be a systemic vasculopathy. This vasculopathy includes characteristic noninflammatory macrovascular and microvascular changes with dramatic and possibly occlusive formation of a thickened neointima. Scleroderma vessels also have an unusual endothelial phenotype, with loss of normal markers including vascular endothelial (VE)-cadherin. These endothelial cells express type 1 interferon and regulator of G protein signaling 5 (RGS5), two molecules associated with vascular rarefaction. These genes may be important because tissue is hypoxic with high levels of vascular endothelial growth factor (VEGF), especially early in the disease. The combination of VEGF and rarefaction is not necessarily paradoxical. VEGF-mediated angiogenesis creates labile vessels that may not survive unless the vessel acquires a smooth muscle coat. The combination of interferon and RGS5 is consistent with an antiangiogenic phenotype. We offer a hypothesis that places vascular injury at the center of this disease and also suggest possible clinical approaches for arresting and/or reversing the disease.
Similar content being viewed by others
References and Recommended Reading
LeRoy EC: Systemic sclerosis. A vascular perspective. Rheum Dis Clin North Am 1996, 22:675–694.
Maricq HR: Capillary abnormalities, Raynaud’s phenomenon, and systemic sclerosis in patients with localized scleroderma. Arch Dermatol 1992, 128:630–632.
Carpentier PH, Satger B, Poensin D, Maricq HR: Incidence and natural history of Raynaud phenomenon: a long-term follow-up (14 years) of a random sample from the general population. J Vasc Surg 2006, 44:1023–1028.
Iwata H, Sata M: Potential contribution of bone marrow-derived precursors to vascular repair and lesion formation: lessons from animal models of vascular diseases. Front Biosci 2007, 12:4157–4167.
Passman JN, Dong XR, Wu SP, et al.: A Sonic hedgehog signaling domain in the arterial adventitia supports resident sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A 2008, 105:9349–9354.
D’Angelo WA, Fries JF, Masi AT, Shulman LE: Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969, 46:428–440.
Yamagishi M, Miyatake K, Tamai J, et al.: Intravascular ultrasound detection of atherosclerosis at the site of focal vasospasm in angiographically normal or minimally narrowed coronary segments. J Am Coll Cardiol 1994, 23:352–357.
Suzuki H, Kawai S, Aizawa T, et al.: Histological evaluation of coronary plaque in patients with variant angina: relationship between vasospasm and neointimal hyperplasia in primary coronary lesions. J Am Coll Cardiol 1999, 33:198–205.
Slomp J, van Munsteren JC, Poelmann RE, et al.: Formation of intimal cushions in the ductus arteriosus as a model for vascular intimal thickening. An immunohistochemical study of changes in extracellular matrix components. Atherosclerosis 1992, 93:25–39.
Nagy Z, Czirjak L: Nailfold digital capillaroscopy in 447 patients with connective tissue disease and Raynaud’s disease. J Eur Acad Dermatol Venereol 2004, 18:62–68.
Birkenhager R, Schneppe B, Rockl W, et al.: Synthesis and physiological activity of heterodimers comprising different splice forms of vascular endothelial growth factor and placenta growth factor. Biochem J 1996, 316:703–707.
Distler O, Distler JH, Scheid A, et al.: Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res 2004, 95:109–116.
Mulligan-Kehoe MJ, Drinane MC, Mollmark J, et al.: Antiangiogenic plasma activity in patients with systemic sclerosis. Arthritis Rheum 2007, 56:3448–3458.
Jun JB, Kuechle M, Harlan JM, Elkon KB: Fibroblast and endothelial apoptosis in systemic sclerosis. Curr Opin Rheumatol 2003, 15:756–760.
Fleming JN, Nash RA, McLeod DO, et al.: Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS ONE 2008, 3:e1452.
Del Papa N, Colombo G, Fracchiolla N, et al.: Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum 2004, 50:1296–1304.
Leroy EC: The vascular defect in scleroderma (systemic sclerosis). Acta Med Scand Suppl 1987, 715:165–167.
Maricq HR: Comparison of quantitative and semiquantitative estimates of nailfold capillary abnormalities in scleroderma spectrum disorders. Microvasc Res 1986, 32:271–276.
Libby P, Pober JS: Chronic rejection. Immunity 2001, 14:387–397.
Corpechot C, Barbu V, Wendum D, et al.: Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 2002 35:1010–1021.
Sgonc R, Gruschwitz MS, Dietrich H, et al.: Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest 1996, 98:785–792.
Nguyen VA, Sgonc R, Dietrich H, Wick G: Endothelial injury in internal organs of University of California at Davis line 200 (UCD 200) chickens, an animal model for systemic sclerosis (scleroderma). J Autoimmun 2000, 14:143–149.
Rouquette-Gally AM, Boyeldieu D, Gluckman E, et al.: Autoimmunity in 28 patients after allogeneic bone marrow transplantation: comparison with Sjogren syndrome and scleroderma. Br J Haematol 1987, 66:45–47.
Zhang Y, Gilliam AC: Animal models for scleroderma: an update. Curr Rheumatol Rep 2002, 4:150–162.
Siracusa LD, Christner P, McGrath R, et al.: The tight skin (tsk) mutation in the mouse, a model for human fibrotic diseases, is tightly linked to the beta 2-microglobulin (b2m) gene on chromosome 2. Genomics 1993, 17:748–751.
Clark SH: Animal models in scleroderma. Curr Rheumatol Rep 2005, 7:150–155.
Lakos G, Takagawa S, Varga J: Animal models of scleroderma. Methods Mol Med 2004, 102:377–393.
Ahmed SS, Tan FK, Arnett FC, et al.: Induction of apoptosis and fibrillin 1 expression in human dermal endothelial cells by scleroderma sera containing anti-endothelial cell antibodies. Arthritis Rheum 2006, 54:2250–2262.
Kahaleh MB, Fan PS: Mechanism of serum-mediated endothelial injury in scleroderma: identification of a granular enzyme in scleroderma skin and sera. Clin Immunol Immunopathol 1997, 83:32–40.
Nevskaya T, Bykovskaia S, Lyssuk E, et al.: Circulating endothelial progenitor cells in systemic sclerosis: relation to impaired angiogenesis and cardiovascular manifestations. Clin Exp Rheumatol 2008, 26:421–429.
Abraham D, Distler O: How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther 2007, 9(Suppl 2):S2.
Anderegg U, Saalbach A, Haustein UF: Chemokine release from activated human dermal microvascular endothelial cells—implications for the pathophysiology of scleroderma? Arch Dermatol Res 2000, 292:341–347.
Hebbar M, Lassalle P, Janin A, et al.: E-selectin expression in salivary endothelial cells and sera from patients with systemic sclerosis. Role of resident mast cell-derived tumor necrosis factor alpha. Arthritis Rheum 1995, 38:406–412.
Carmeliet P: Angiogenesis in life, disease and medicine. Nature 2005, 438:932–936.
Duan H, Fleming J, Pritchard DK, et al.: Combined analysis of monocyte and lymphocyte messenger rna expression with serum protein profiles in patients with scleroderma. Arthritis Rheum 2008, 58:1465–1474.
Rozera C, Carlei D, Lollini PL, et al.: Interferon (IFN)-beta gene transfer into ts/a adenocarcinoma cells and comparison with IFN-alpha: differential effects on tumorigenicity and host response. Am J Pathol 1999, 154:1211–1222.
Chang E, Boyd A, Nelson CC, et al.: Successful treatment of infantile hemangiomas with interferon-alpha-2b. J Pediatr Hematol Oncol 1997, 19:237–244.
Stout AJ, Gresser I, Thompson WD: Inhibition of wound healing in mice by local interferon alpha/beta injection. Int J Exp Pathol 1993, 74:79–85.
Dor Y, Porat R, Keshet E: Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasis. Am J Physiol Cell Physiol 2001, 280:C1367–C1374.
Cho H, Kozasa T, Bondjers C, et al.: Pericyte-specific expression of RGS5: Implications for PDGF and edg receptor signaling during vascular maturation. FASEB J 2003, 17:440–442.
Iruela-Arispe L: Angiogenesis: novel and basic science insights and human therapy—Keystone symposium. IDrugs 2004, 7:111–113.
Berger M, Bergers G, Arnold B, et al.: Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood 2005, 105:1094–101.
Sela S, Itin A, Natanson-Yaron S, et al.: A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res 2008, 102:1566–1574.
Torsney E, Hu Y, Xu Q: Adventitial progenitor cells contribute to arteriosclerosis. Trends Cardiovasc Med 2005, 15:64–68.
Lavine KJ, Kovacs A, Ornitz DM: Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest 2008, 118:2404–2414.
Lavine KJ, Long F, Choi K, et al.: Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development 2008, 135:3161–3171.
Sas A, Jones R, Tyor W: Intra-peritoneal injection of polyclonal anti-interferon alpha antibodies cross the blood brain barrier and neutralize interferon alpha. Neurochem Res 2008, 33:2281–2287.
Nash RA, Bowen JD, McSweeney PA, et al.: High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood 2003, 102:2364–2372.
Kim D, Peck A, Santer D, et al.: Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum 2008, 58:2163–2173.
Higgins DF, Kimura K, Iwano M, Haase VH: Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle 2008, 7:1128–1132.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fleming, J.N., Nash, R.A., Mahoney, W.M. et al. Is scleroderma a vasculopathy?. Curr Rheumatol Rep 11, 103–110 (2009). https://doi.org/10.1007/s11926-009-0015-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-009-0015-3