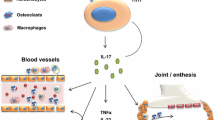
Abstract
As our understanding of the pathogenesis of autoimmune diseases is growing, new therapies are being developed to target disease-specific pathways. Since the introduction of etanercept in 1998, several biotechnological agents have been developed, most of them indicated in the treatment of rheumatoid arthritis, but also psoriatic arthritis. Most currently available molecules target TNF-alfa with different strategies (i.e., etanercept, infliximab, adalimumab, golimumab, and certolizumab pegol), IL-6 (tocilizumab), CTLA-4 (abatacept), and B cells (rituximab, belimumab) as they are key mediators in the cascade of inflammation. Further, small molecules have been recently developed to target intracellular signaling, such as Janus Kinases for tofacitinib, the first FDA-approved small molecule for rheumatoid arthritis. Most novel treatments are being developed for arthritis with specific differences between rheumatoid and psoriatic arthritis, as well as for systemic lupus erythematosus, following the approval of belimumab. Finally, biologic therapies are effective also in gout, mainly targeting interleukin-1 to block the inflammasome. This review article describes the new and upcoming treatment options for rheumatoid arthritis, psoriatic arthritis, systemic lupus erythematosus, and gout to dissect what we should be aware of when discussing these new and promising molecules.
Similar content being viewed by others
References
Choy EH, Kavanaugh AF, Jones SA. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol. 2013;9(3):154–63. doi:10.1038/nrrheum.2013.8.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19. doi:10.1056/NEJMra1004965. doi:10.7748/phc2011.11.21.9.29.c8797.
Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford, England). 2009;48(10):1309–13. doi:10.1093/rheumatology/kep252.
Gabriel SE. Heart disease and rheumatoid arthritis: understanding the risks. Ann Rheum Dis. 2010;69(Suppl 1):i61–4. doi:10.1136/ard.2009.119404.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi:10.1002/art.27584.
Gladman DD. Psoriatic arthritis. Dermatol Ther. 2009;22(1):40–55. doi:10.1111/j.1529-8019.2008.01215.x.
Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11(3):229. doi:10.1186/ar2669.
Duarte GV, Faillace C, Freire de Carvalho J. Psoriatic arthritis. Best Pract Res Clin Rheumatol. 2012;26(1):147–56. doi:10.1016/j.berh.2012.01.003.
Taylor WJ, Helliwell PS. Development of diagnostic criteria for psoriatic arthritis: methods and process. Curr Rheumatol Rep. 2004;6(4):299–305.
Chimenti MS, Ballanti E, Perricone C, Cipriani P, Giacomelli R, Perricone R. Immunomodulation in psoriatic arthritis: focus on cellular and molecular pathways. Autoimmun Rev. 2013;12(5):599–606. doi:10.1016/j.autrev.2012.10.002.
Conigliaro P, Scrivo R, Valesini G, Perricone R. Emerging role for NK cells in the pathogenesis of inflammatory arthropathies. Autoimmun Rev. 2011;10(10):577–81. doi:10.1016/j.autrev.2011.04.017.
Lories RJ, de Vlam K. Is psoriatic arthritis a result of abnormalities in acquired or innate immunity? Curr Rheumatol Rep. 2012;14(4):375–82. doi:10.1007/s11926-012-0257-3.
Kirkham BW, Kavanaugh A, Reich K. Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology. 2014;141(2):133–42. doi:10.1111/imm.12142.
Scarpa R. New insights into the concept of psoriatic disease. J Rheumatol Suppl. 2012;89:4–6. doi:10.3899/jrheum.120231.
Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;. doi:10.1016/s0140-6736(14)60128-8.
Cozzani E, Drosera M, Gasparini G. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects. Autoimmun Dis. 2014;2014:321359. doi:10.1155/2014/321359.
Mak A, Kow NY. The pathology of T cells in systemic lupus erythematosus. J Immunol Res. 2014;2014:419029. doi:10.1155/2014/419029.
Peng H, Wang W, Zhou M, Li R, Pan HF, Ye DQ. Role of interleukin-10 and interleukin-10 receptor in systemic lupus erythematosus. Clin Rheumatol. 2013;32(9):1255–66. doi:10.1007/s10067-013-2294-3.
Candon S, Gottenberg JE, Bengoufa D, Chatenoud L, Mariette X. Quantitative assessment of antibodies to ribonucleoproteins in primary Sjogren syndrome: correlation with B-cell biomarkers and disease activity. Ann Rheum Dis. 2009;68(7):1208–12. doi:10.1136/ard.2008.095257.
Pers JO, Youinou P. Are the B cells cast with the leading part in the Sjogren’s syndrome scenario? Oral Dis. 2013;. doi:10.1111/odi.12153.
Voulgarelis M, Tzioufas AG. Current aspects of pathogenesis in Sjogren’s Syndrome. Ther Adv Musculoskelet Dis. 2010;2(6):325–34. doi:10.1177/1759720x10381431.
Leandro MJ, Cambridge G. Expression of B cell activating factor (BAFF) and BAFF-binding receptors in rheumatoid arthritis. J Rheumatol. 2013;40(8):1247–50. doi:10.3899/jrheum.130677.
Moura RA, Canhao H, Polido-Pereira J, Rodrigues AM, Navalho M, Mourao AF, et al. BAFF and TACI gene expression are increased in patients with untreated very early rheumatoid arthritis. J Rheumatol. 2013;40(8):1293–302. doi:10.3899/jrheum.121110.
Scholz JL, Oropallo MA, Sindhava V, Goenka R, Cancro MP. The role of B lymphocyte stimulator in B cell biology: implications for the treatment of lupus. Lupus. 2013;22(4):350–60. doi:10.1177/0961203312469453.
Juraschek SP, Miller ER 3rd, Gelber AC. Body mass index, obesity, and prevalent gout in the United States in 1988–1994 and 2007–2010. Arthritis Care Res. 2013;65(1):127–32. doi:10.1002/acr.21791.
Roddy E, Mallen CD, Doherty M. Gout. BMJ (Clinical Research ed). 2013;347:f5648. doi:10.1136/bmj.f5648.
Horton SC, Emery P. Biological therapy for rheumatoid arthritis: where are we now? British J Hosp Med (Lond Engl: 2005). 2012;73(1):12–8.
Dorner T, Strand V, Castaneda-Hernandez G, Ferraccioli G, Isaacs JD, Kvien TK, et al. The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis. 2013;72(3):322–8. doi:10.1136/annrheumdis-2012-202715.
Fleischmann R. Novel small-molecular therapeutics for rheumatoid arthritis. Curr Opin Rheumatol. 2012;24(3):335–41. doi:10.1097/BOR.0b013e32835190ef.
Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72(6):863–9. doi:10.1136/annrheumdis-2012-201601.
Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Aelion JA, et al. One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled study. J Rheumatol. 2014;41(3):414–21. doi:10.3899/jrheum.130637.
Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62(4):929–39. doi:10.1002/art.27334.
Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS et al. A Phase II Randomized Study of Subcutaneous Ixekizumab, an Anti-Interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumat (Hoboken, NJ). 2014;66(7):1693–704. doi:10.1002/art.38617.
Martin DA, Churchill M, Flores-Suarez L, Cardiel MH, Wallace D, Martin R, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R164. doi:10.1186/ar4347.
Krausz S, Boumans MJ, Gerlag DM, Lufkin J, van Kuijk AW, Bakker A, et al. Brief report: a phase IIa, randomized, double-blind, placebo-controlled trial of apilimod mesylate, an interleukin-12/interleukin-23 inhibitor, in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(6):1750–5. doi:10.1002/art.34339.
Bugatti S, Vitolo B, Caporali R, Montecucco C, Manzo A. B cells in rheumatoid arthritis: from pathogenic players to disease biomarkers. BioMed Res Int. 2014;2014:681678. doi:10.1155/2014/681678.
Mei HE, Schmidt S, Dorner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res Ther. 2012;14(Suppl 5):S1. doi:10.1186/ar3909.
Genovese MC, Kaine JL, Lowenstein MB, Del Giudice J, Baldassare A, Schechtman J, et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58(9):2652–61. doi:10.1002/art.23732.
Emery P, Rigby W, Tak PP, Dorner T, Olech E, Martin C, et al. Safety with ocrelizumab in rheumatoid arthritis: results from the ocrelizumab phase III program. PLoS One. 2014;9(2):e87379. doi:10.1371/journal.pone.0087379.
Harigai M, Tanaka Y, Maisawa S. Safety and efficacy of various dosages of ocrelizumab in Japanese patients with rheumatoid arthritis with an inadequate response to methotrexate therapy: a placebo-controlled double-blind parallel-group study. J Rheumatol. 2012;39(3):486–95. doi:10.3899/jrheum.110994.
Rigby W, Tony HP, Oelke K, Combe B, Laster A, von Muhlen CA, et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum. 2012;64(2):350–9. doi:10.1002/art.33317.
Stohl W, Gomez-Reino J, Olech E, Dudler J, Fleischmann RM, Zerbini CA, et al. Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial. Ann Rheum Dis. 2012;71(8):1289–96. doi:10.1136/annrheumdis-2011-200706.
Tak PP, Mease PJ, Genovese MC, Kremer J, Haraoui B, Tanaka Y, et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to at least one tumor necrosis factor inhibitor: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum. 2012;64(2):360–70.
Kurrasch R, Brown JC, Chu M, Craigen J, Overend P, Patel B, et al. Subcutaneously administered ofatumumab in rheumatoid arthritis: a phase I/II study of safety, tolerability, pharmacokinetics, and pharmacodynamics. J Rheumatol. 2013;40(7):1089–96. doi:10.3899/jrheum.121118.
Ostergaard M, Baslund B, Rigby W, Rojkovich B, Jorgensen C, Dawes PT, et al. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum. 2010;62(8):2227–38. doi:10.1002/art.27524.
Taylor PC, Quattrocchi E, Mallett S, Kurrasch R, Petersen J, Chang DJ. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis. 2011;70(12):2119–25. doi:10.1136/ard.2011.151522.
Stohl W, Merrill JT, McKay JD, Lisse JR, Zhong ZJ, Freimuth WW, et al. Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled, dose-ranging Study. J Rheumatol. 2013;40(5):579–89. doi:10.3899/jrheum.120886.
Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, et al. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 2008;58(1):61–72. doi:10.1002/art.23178.
Genovese MC, Kinnman N, de La Bourdonnaye G, Pena Rossi C, Tak PP. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum. 2011;63(7):1793–803. doi:10.1002/art.30373.
van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1782–92. doi:10.1002/art.30372.
Genovese MC, Bojin S, Biagini IM, Mociran E, Cristei D, Mirea G, et al. Tabalumab in rheumatoid arthritis patients with an inadequate response to methotrexate and naive to biologic therapy: a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 2013;65(4):880–9. doi:10.1002/art.37820.
Genovese MC, Fleischmann RM, Greenwald M, Satterwhite J, Veenhuizen M, Xie L, et al. Tabalumab, an anti-BAFF monoclonal antibody, in patients with active rheumatoid arthritis with an inadequate response to TNF inhibitors. Ann Rheum Dis. 2013;72(9):1461–8. doi:10.1136/annrheumdis-2012-202775.
Genovese MC, Lee E, Satterwhite J, Veenhuizen M, Disch D, Berclaz PY, et al. A phase 2 dose-ranging study of subcutaneous tabalumab for the treatment of patients with active rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2013;72(9):1453–60. doi:10.1136/annrheumdis-2012-202864.
Woodrick RS, Ruderman EM. IL-6 inhibition for the treatment of rheumatoid arthritis and other conditions. Bull NYU Hosp Joint Dis. 2012;70(3):195–9.
Huizinga TW, Fleischmann RM, Jasson M, Radin AR, van Adelsberg J, Fiore S, et al. Sarilumab, a fully human monoclonal antibody against IL-6Ralpha in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. 2013;. doi:10.1136/annrheumdis-2013-204405.
Smolen JS, Weinblatt ME, Sheng S, Zhuang Y, Hsu B. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2014;. doi:10.1136/annrheumdis-2013-205137.
Geyer M, Muller-Ladner U. Actual status of antiinterleukin-1 therapies in rheumatic diseases. Curr Opin Rheumatol. 2010;22(3):246–51. doi:10.1097/BOR.0b013e3283373fa0.
Ruckert R, Brandt K, Ernst M, Marienfeld K, Csernok E, Metzler C, et al. Interleukin-15 stimulates macrophages to activate CD4 + T cells: a role in the pathogenesis of rheumatoid arthritis? Immunology. 2009;126(1):63–73. doi:10.1111/j.1365-2567.2008.02878.x.
Hintzen C, Quaiser S, Pap T, Heinrich PC, Hermanns HM. Induction of CCL13 expression in synovial fibroblasts highlights a significant role of oncostatin M in rheumatoid arthritis. Arthritis Rheum. 2009;60(7):1932–43. doi:10.1002/art.24602.
Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford, England). 2004;43(Suppl 3):iii2–9. doi:10.1093/rheumatology/keh201.
Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database of Syst Rev. 2009(1):Cd005121. doi:10.1002/14651858.CD005121.pub3.
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64(5):625–39. doi:10.1002/acr.21641.
Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117(20):2662–9. doi:10.1161/circulationaha.107.731877.
Ljung L, Olsson T, Engstrand S, Wallberg-Jonsson S, Soderberg S, Rantapaa-Dahlqvist S. Interleukin-1 receptor antagonist is associated with both lipid metabolism and inflammation in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25(4):617–20.
Chakraborty A, Tannenbaum S, Rordorf C, Lowe PJ, Floch D, Gram H, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1beta monoclonal antibody. Clin Pharmacokinet. 2012;51(6):e1–18. doi:10.2165/11599820-000000000-00000.
Alten R, Gomez-Reino J, Durez P, Beaulieu A, Sebba A, Krammer G, et al. Efficacy and safety of the human anti-IL-1beta monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, Phase II, dose-finding study. BMC Musculoskelet Disord. 2011;12:153. doi:10.1186/1471-2474-12-153.
Masuko-Hongo K, Kurokawa M, Kobata T, Nishioka K, Kato T. Effect of IL15 on T cell clonality in vitro and in the synovial fluid of patients with rheumatoid arthritis. Ann Rheum Dis. 2000;59(9):688–94.
Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52(9):2686–92. doi:10.1002/art.21249.
Choy EH, Bendit M, McAleer D, Liu F, Feeney M, Brett S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of an anti- oncostatin M monoclonal antibody in rheumatoid arthritis: results from phase II randomized, placebo-controlled trials. Arthritis Res Ther. 2013;15(5):R132. doi:10.1186/ar4312.
Keystone E, Cohen MD. Cell-signaling therapy in rheumatoid arthritis. Curr Rheumatol Rep. 2013;15(10):368. doi:10.1007/s11926-013-0368-5.
Migita K, Izumi Y, Torigoshi T, Satomura K, Izumi M, Nishino Y, et al. Inhibition of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway in rheumatoid synovial fibroblasts using small molecule compounds. Clin Exp Immunol. 2013;174(3):356–63. doi:10.1111/cei.12190.
Kawalec P, Mikrut A, Wisniewska N, Pilc A. The effectiveness of tofacitinib, a novel Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2013;32(10):1415–24. doi:10.1007/s10067-013-2329-9.
Shi JG, Chen X, Lee F, Emm T, Scherle PA, Lo Y, et al. The pharmacokinetics, pharmacodynamics and safety of Baricitinib, an oral JAK 1/2 Inhibitor, in Healthy Volunteers. J Clin Pharmacol. 2014;. doi:10.1002/jcph.354.
Mesa RA. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs Investig Drugs J. 2010;13(6):394–403.
Gan EY, Chong WS, Tey HL. Therapeutic strategies in psoriasis patients with psoriatic arthritis: focus on new agents. BioDrugs Clin Immunother Biopharm Gene Ther. 2013;27(4):359–73. doi:10.1007/s40259-013-0025-6.
Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–6. doi:10.1136/annrheumdis-2013-205056.
Palfreeman AC, McNamee KE, McCann FE. New developments in the management of psoriasis and psoriatic arthritis: a focus on apremilast. Drug Des Dev Ther. 2013;7:201–10. doi:10.2147/dddt.s32713.
Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64(10):3156–67. doi:10.1002/art.34627.
Wittmann M, Helliwell PS. Phosphodiesterase 4 inhibition in the treatment of psoriasis, psoriatic arthritis and other chronic inflammatory diseases. Dermatol Ther. 2013;3(1):1–15. doi:10.1007/s13555-013-0023-0.
Schett G, Sloan VS, Stevens RM, Schafer P. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther Adv Musculoskelet Dis. 2010;2(5):271–8. doi:10.1177/1759720x10381432.
Kim HR, Kim KW, Kim BM, Jung HG, Cho ML, Lee SH. Reciprocal activation of CD4+ T cells and synovial fibroblasts by stromal cell-derived factor 1 promotes RANKL expression and osteoclastogenesis in rheumatoid arthritis. Arthritis Rheumatol (Hoboken, NJ). 2014;66(3):538–48. doi:10.1002/art.38286.
Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, et al. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunol. 2003;170(2):838–45.
Filer A. The fibroblast as a therapeutic target in rheumatoid arthritis. Curr Opin Pharmacol. 2013;13(3):413–9. doi:10.1016/j.coph.2013.02.006.
Kiener HP, Niederreiter B, Lee DM, Jimenez-Boj E, Smolen JS, Brenner MB. Cadherin 11 promotes invasive behavior of fibroblast-like synoviocytes. Arthritis Rheum. 2009;60(5):1305–10. doi:10.1002/art.24453.
Bauer S, Jendro MC, Wadle A, Kleber S, Stenner F, Dinser R, et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther. 2006;8(6):R171. doi:10.1186/ar2080.
Ospelt C, Mertens JC, Jungel A, Brentano F, Maciejewska-Rodriguez H, Huber LC, et al. Inhibition of fibroblast activation protein and dipeptidylpeptidase 4 increases cartilage invasion by rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2010;62(5):1224–35. doi:10.1002/art.27395.
El-Jawhari JJ, El-Sherbiny YM, Jones EA, McGonagle D. Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM Mon J Assoc Physicians. 2014;107(7):505–14. doi:10.1093/qjmed/hcu033.
Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future directions. Expert Opin Pharmacother. 2013;14(13):1755–64. doi:10.1517/14656566.2013.810208.
Gossec L, Smolen JS, Gaujoux-Viala C, Ash Z, Marzo-Ortega H, van der Heijde D, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis. 2012;71(1):4–12. doi:10.1136/annrheumdis-2011-200350.
Papoutsaki M, Costanzo A. Treatment of psoriasis and psoriatic arthritis. BioDrugs Clin Immunother Biopharm Gene Ther. 2013;27(Suppl 1):3–12. doi:10.1007/bf03325637.
Jimenez-Boj E, Stamm TA, Sadlonova M, Rovensky J, Raffayova H, Leeb B, et al. Rituximab in psoriatic arthritis: an exploratory evaluation. Ann Rheum Dis. 2012;71(11):1868–71. doi:10.1136/annrheumdis-2012-201897.
Altmeyer MD, Kerisit KG, Boh EE. Therapeutic hotline. Abatacept: our experience of use in two patients with refractory psoriasis and psoriatic arthritis. Dermatol Ther. 2011;24(2):287–90. doi:10.1111/j.1529-8019.2011.01405.x.
Rodrigues CE, Vieira FJ, Callado MR, Gomes KW, de Andrade JE, Vieira WP. Use of the abatacept in a patient with psoriatic arthritis. Revista Brasileira de Reumatologia. 2010;50(3):340–5.
Ursini F, Naty S, Russo E, Grembiale RD. Abatacept in psoriatic arthritis: case report and short review. J Pharmacol Pharmacother. 2013;4(Suppl 1):S29–32. doi:10.4103/0976-500x.120943.
Vieira FJ, Callado MR, Vieira WP. Abatacept as an option therapy in difficult to treat psoriatic arthritis. Rheumatol Int. 2010;30(6):849–50. doi:10.1007/s00296-009-1041-1.
Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63(4):939–48. doi:10.1002/art.30176.
Cauli A, Mathieu A. Th17 and interleukin 23 in the pathogenesis of psoriatic arthritis and spondyloarthritis. J Rheumatol Suppl. 2012;89:15–8. doi:10.3899/jrheum.120234.
Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13(4–5):496–502. doi:10.1016/j.autrev.2014.01.050.
Zhu KJ, Zhu CY, Shi G, Fan YM. Meta-analysis of IL12B polymorphisms (rs3212227, rs6887695) with psoriasis and psoriatic arthritis. Rheumatol Int. 2013;33(7):1785–90. doi:10.1007/s00296-012-2637-4.
Catanoso MG, Boiardi L, Macchioni P, Garagnani P, Sazzini M, De Fanti S, et al. IL-23A, IL-23R, IL-17A and IL-17R polymorphisms in different psoriatic arthritis clinical manifestations in the northern Italian population. Rheumatol Int. 2013;33(5):1165–76. doi:10.1007/s00296-012-2501-6.
McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–9. doi:10.1016/s0140-6736(13)60594-2.
Kavanaugh A, Ritchlin C, Rahman P, Puig L, Gottlieb AB, Li S, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73(6):1000–6. doi:10.1136/annrheumdis-2013-204741.
Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990–9. doi:10.1136/annrheumdis-2013-204655.
Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis. 2013;72 (Suppl 2):ii116–23. doi:10.1136/annrheumdis-2012-202371.
McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73(2):349–56. doi:10.1136/annrheumdis-2012-202646.
Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38. doi:10.1056/NEJMoa1314258.
Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–306. doi:10.1056/NEJMoa1315231.
Zouali M, Uy EA. Belimumab therapy in systemic lupus erythematosus. BioDrugs Clin Immunother Biopharm Gene Ther. 2013;27(3):225–35. doi:10.1007/s40259-013-0031-8.
Cobo-Ibanez T, Loza-Santamaria E, Pego-Reigosa JM, Marques AO, Rua-Figueroa I, Fernandez-Nebro A, et al. Efficacy and safety of rituximab in the treatment of non-renal systemic lupus erythematosus: a systematic review. Semin Arthritis Rheum. 2014;. doi:10.1016/j.semarthrit.2014.04.002.
Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71(11):1771–82. doi:10.1136/annrheumdis-2012-201940.
Reddy V, Jayne D, Close D, Isenberg D. B-cell depletion in SLE: clinical and trial experience with rituximab and ocrelizumab and implications for study design. Arthritis Res Ther. 2013;15(Suppl 1):S2. doi:10.1186/ar3910.
Daridon C, Blassfeld D, Reiter K, Mei HE, Giesecke C, Goldenberg DM, et al. Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12(6):R204. doi:10.1186/ar3179.
Pena-Rossi C, Nasonov E, Stanislav M, Yakusevich V, Ershova O, Lomareva N, et al. An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus. Lupus. 2009;18(6):547–55. doi:10.1177/0961203309102803.
Isenberg D, Gordon C, Licu D, Copt S, Rossi CP, Wofsy D. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis. 2014;. doi:10.1136/annrheumdis-2013-205067.
Merrill JT. Co-stimulatory molecules as targets for treatment of lupus. Clin Immunol (Orlando, FL). 2013;148(3):369–75. doi:10.1016/j.clim.2013.04.012.
Merrill JT, Burgos-Vargas R, Westhovens R, Chalmers A, D’Cruz D, Wallace DJ, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(10):3077–87. doi:10.1002/art.27601.
Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62(2):542–52. doi:10.1002/art.27221.
Xu Z, Bouman-Thio E, Comisar C, Frederick B, Van Hartingsveldt B, Marini JC, et al. Pharmacokinetics, pharmacodynamics and safety of a human anti-IL-6 monoclonal antibody (sirukumab) in healthy subjects in a first-in-human study. Br J Clin Pharmacol. 2011;72(2):270–81. doi:10.1111/j.1365-2125.2011.03964.x.
O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111–5. doi:10.1136/annrheumdis-2012-202576.
Sthoeger Z, Sharabi A, Mozes E. Novel approaches to the development of targeted therapeutic agents for systemic lupus erythematosus. J Autoimmun. 2014;. doi:10.1016/j.jaut.2014.06.002.
Crittenden DB, Pillinger MH. New therapies for gout. Annu Rev Med. 2013;64:325–37. doi:10.1146/annurev-med-080911-105830.
So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28. doi:10.1186/ar2143.
Schlesinger N, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat Gouty Arthritis by suppressing inflammation: results of a randomized, dose-ranging study. Arthritis Res Ther. 2011;13(2):R53. doi:10.1186/ar3297.
Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012;71(11):1839–48. doi:10.1136/annrheumdis-2011-200908.
So A, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010;62(10):3064–76. doi:10.1002/art.27600.
Schlesinger N, Mysler E, Lin HY, De Meulemeester M, Rovensky J, Arulmani U, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis. 2011;70(7):1264–71. doi:10.1136/ard.2010.144063.
Schumacher HR Jr, Evans RR, Saag KG, Clower J, Jennings W, Weinstein SP, et al. Rilonacept (interleukin-1 trap) for prevention of gout flares during initiation of uric acid-lowering therapy: results from a phase III randomized, double-blind, placebo-controlled, confirmatory efficacy study. Arthritis Care Res. 2012;64(10):1462–70. doi:10.1002/acr.21690.
Sundy JS, Schumacher HR, Kivitz A, Weinstein SP, Wu R, King-Davis S, et al. Rilonacept for gout flare prevention in patients receiving uric acid-lowering therapy: results of RESURGE, a Phase III, international safety study. J Rheumatol. 2014;. doi:10.3899/jrheum.131226.
Goh AX, Bertin-Maghit S, Ping Yeo S, Ho AW, Derks H, Mortellaro A et al. A novel human anti-interleukin-1beta neutralizing monoclonal antibody showing in vivo efficacy. mAbs. 2014;6(3):765–73. doi:10.4161/mabs.28614.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selmi, C., Generali, E., Massarotti, M. et al. New treatments for inflammatory rheumatic disease. Immunol Res 60, 277–288 (2014). https://doi.org/10.1007/s12026-014-8565-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-014-8565-5