Abstract
Women with pulmonary hypertension have a high risk of morbidity and mortality during pregnancy. The inability to increase cardiac output leads to heart failure while further risks are introduced with hypercoagulability and decrease in systemic vascular resistance. There is no proof that new advanced therapies for pulmonary hypertension decrease the risk, though some promising results have been reported. However, pregnancy should still be regarded as contraindicated in women with pulmonary hypertension. When pregnancy occurs and termination is declined, pregnancy and delivery should be managed by multidisciplinary services with experience in the management of both pulmonary hypertension and high-risk pregnancies.
Similar content being viewed by others
Introduction
Pulmonary hypertension is a rare disorder that can be present in women of child-bearing age. During pregnancy it is associated with high morbidity and mortality in all defined clinical groups of pulmonary hypertension (Table 1). Therefore, pulmonary hypertension is regarded a contraindication for pregnancy [1–4]. However, sometimes women become pregnant despite being advised against pregnancy, or pulmonary hypertension is newly diagnosed during pregnancy. These women are usually advised to terminate the pregnancy even though termination itself is also associated with maternal risks. Some women do not regard termination as an acceptable option and carry on with their pregnancy. This special article discusses the definition, classification, pathophysiology and clinical features of pulmonary hypertension during pregnancy, it reviews the literature on outcome and covers the management of pregnancy in women with pulmonary hypertension, including the effects of advanced anti-pulmonary hypertension therapies.
Definition and classification
Pulmonary hypertension is defined as an increase in mean pulmonary arterial pressure (mPAP) ≥25 mmHg at rest as assessed by right heart catheterisation [2]. It can be present in multiple clinical conditions. The clinical classification of pulmonary hypertension is summarised in Table 1 [2]. Haemodynamically, group 1, 3, 4, and 5 are characterised by a pulmonary wedge pressure <15 mmHg and are referred to as pre-capillary pulmonary hypertension, while patients in group 2 have postcapillary pulmonary hypertension with a pulmonary wedge pressure >15 mmHg. The histopathology differs between the clinical groups and can include medial hypertrophy, intimal proliferation, fibrosis, thrombotic lesions and venous thickening. Complex plexiform lesions are only present in group 1 (pulmonary arterial hypertension). Vasoconstriction, obstruction, vascular remodelling and endothelial dysfunction contribute to the increase in pulmonary vascular resistance [2, 5].
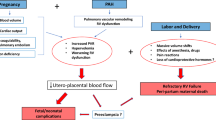
Pathophysiology related to pregnancy
The normal physiological changes of pregnancy are poorly tolerated by women with pulmonary hypertension. Pregnancy is associated with increased plasma volume and decreased systemic vascular resistance, both resulting in an increase in cardiac output. In healthy women a decrease in pulmonary vascular resistance accommodates the requirements for increased cardiac output. In women with pulmonary hypertension, pulmonary vascular disease prevents the fall in pulmonary vascular resistance, leading to a rise in pulmonary artery pressure with increased cardiac output. Ultimately, the necessary increase in cardiac output can not be achieved resulting in right heart failure. The leftward shift of the interventricular septum increases, impairing diastolic filling of the left ventricle and further compromising cardiac output. Moreover, the hypercoagulability of pregnancy increases the risk of pulmonary emboli and pulmonary arterial thrombosis. Paradoxical emboli are a risk in women with a patent foramen ovale or Eisenmenger syndrome. Women with Eisenmenger syndrome are at particular risk of increased right-to-left shunting due to the decrease in systemic vascular resistance, which results in increased hypoxia, thus aggravating pulmonary vasoconstriction and right heart failure [5, 6]. Hypoxia can also lead to syncope and sudden death in this group of patients.
During labour and delivery the risk of complications increases due to volume shifts resulting from blood loss and uterus contractions, vasovagal reaction to pain, acidosis and hypercarbia leading to a further increase in pulmonary vascular resistance, and an increased risk of thromboembolic complications.
Clinical features and diagnosis
Fatigue and exertional dyspnoea are the most frequent presenting symptoms. They are due to reduced cardiac output and impaired oxygen transport. Since these symptoms also occur in healthy pregnant women, the diagnosis during pregnancy can be delayed. Usually the symptoms aggravate during the course of pregnancy and dyspnoea can occur at rest. Chest pain is often present and reflects right ventricular ischaemia. Syncope can result from low cardiac output. Several signs of right heart failure, such as hepatomegaly, ascites, and ankle oedema, may be hard to identify during pregnancy or resemble normal pregnancy. Elevated jugular venous pressure and a loud pulmonary component of the second heart sound are useful signs that point to the diagnosis. Echocardiography will usually reveal the diagnosis. The threshold to perform echocardiography should be low during pregnancy in women with dyspnoea [1]. Right heart catheterisation is required to confirm the diagnosis and gives useful additional information on pulmonary vascular resistance and cardiac output. It can be performed with relatively low foetal risk since radiation can be avoided. During pregnancy it should be reserved for women in whom the results have therapeutic consequences, because of the associated risks of thromboembolism and infection. Functional capacity needs to be evaluated (New York Heart Association or World Health Organization functional class [2]) and exercise capacity can be assessed with an un-encouraged six-minute walking test.
Prognosis of pregnancy in women with pulmonary hypertension
In two large series identifying predictors of maternal cardiac complications during pregnancy in women with heart disease, pulmonary hypertension did not emerge as a predictor of adverse outcome [7, 8]. This is probably due to low prevalence since women with pulmonary hypertension are generally advised against pregnancy. Based on disease-specific literature, there is no doubt that pulmonary hypertension is associated with severe maternal complications and high mortality. Two systematic literature reviews have described the outcome of pregnancy in women with pulmonary hypertension, covering a total period of almost 30 years and comprising 198 pregnancies [9, 10]. The mortality of women with Eisenmenger syndrome (N = 102) was 36% in the first review (1978–1996) and 28% in the second review (1997–2007). Most women died in the first month after delivery and the main causes of death were heart failure and sudden death, while pulmonary thromboembolism was another frequent cause. In women with idiopathic pulmonary hypertension (N = 56) mortality was 30% in the first and 17% in the second review. Again most women died after delivery and heart failure was an important cause of mortality. Women with other causes of pulmonary hypertension (N = 40) had a mortality of 56% and 33% respectively. Nearly all fatalities occurred early post-partum and death was mainly due to heart failure while sudden death and thromboembolism also contributed. Total mortality was significantly higher in the earlier period (1978–1996) compared with the last 10 years (1997–2007), 38% versus 25% (p = 0.047). However, since many case reports or very small series were included in the reviews, publication bias can not be excluded. In the first review (1978–1996), late diagnosis of pulmonary hypertension (p = 0.002, odds ratio 5.4) and late hospital admission (p = 0.001, odds ratio 1.1 per week of pregnancy) were independent predictors of maternal mortality. In the second review (1997–2007), primigravidae and women who received general anaesthesia at delivery had a higher risk of death. Women with previous pregnancies may have had a lower risk because they had less severe disease, since they have already survived a pregnancy. The higher risk for women who had general anaesthesia may be due to the inherent risks of general anaesthesia, or because general anaesthesia was more often applied in women with a more grave clinical condition [10].
It is not known if women with lower pulmonary pressures and resistance are at lower risk. Systolic or mean pulmonary artery pressures did not predict mortality in either of the reviews, but this is possibly explained by missing data. In the first review (1978–1996) diastolic pulmonary artery pressure was a univariate risk factor for adverse outcome. In both reviews, the majority of women who died had severe pulmonary hypertension, but also some women with mild or moderate pulmonary hypertension deteriorated. A safe cut-off value is not known [1, 2, 9, 10].
In the period covered by the second review, advanced therapy for pulmonary hypertension was becoming increasingly available, but though a significant percentage of women were treated with NO or prostacyclin analogues, the institution of advanced therapy did not predict a better outcome. The authors comment that advanced therapy was in most cases commenced late in the course of the disease, when patients were unstable or had refractory heart failure [10], and that an earlier start of these therapies will possibly improve outcome. Until recently, no evidence concerning this subject was available. In 2010 a single-centre series of 10 consecutive pregnancies during 2002–2009 reported improved outcomes. A multiprofessional approach was applied to all women, with early institution of targeted therapy (nebulised prostanoid therapy, changing to intravenous prostanoid when clinical deterioration or insufficient response occurred). Planned caesarean delivery with epidural or combined epidural/spinal anaesthesia was performed at 34 weeks, or earlier when clinical deterioration occurred. Most women received therapeutic or prophylactic anticoagulation therapy. All 10 pregnancies resulted in live births, and no maternal mortality occurred during pregnancy or early after delivery. One woman died 4 weeks after delivery, after stopping her therapy at home and declining hospital admission when she deteriorated. This series is small, but the results are promising. The authors state that the risk of pregnancy remains high and despite their results, women with pulmonary hypertension should be advised against pregnancy and when they become pregnant, termination should be offered [11.]
Neonatal outcome is also compromised in women with pulmonary hypertension. The rate of premature labour and premature delivery is high. Offspring mortality occurs in about 10%, and seems higher in Eisenmenger syndrome [9, 10, 12]. In Eisenmenger syndrome, there is also a high percentage of small for gestational age children [12].
Management of pregnancy and delivery
Women with pulmonary hypertension should be advised against pregnancy [1–4]. Adequate contraception should be installed. Barrier methods are safe but not sufficiently effective. Low-dose combined oral contraceptives containing ethinyl oestradiol are not suitable for women with pulmonary hypertension since they are thrombogenic. Progesterone-only pills or dermal implants can be used. Endothelin receptor antagonists such as bosentan can reduce the efficacy of oral contraceptives.
The levonorgestrel-releasing intra-uterine device is very effective and safe. Vasovagal reactions can, however, occur during implantation and may be poorly tolerated; therefore, women with pulmonary hypertension should have these devices implanted in hospital [1–3].
When pregnancy occurs, termination should be offered even if the woman is in a good clinical condition. Pregnancy termination is a high-risk procedure and it should be performed in an experienced centre [1, 2].
When the woman chooses to continue pregnancy, it is important that she is managed by a multidisciplinary team in a centre licensed for the treatment of pulmonary hypertension patients. The team should include a pulmonary hypertension specialist, a cardiologist, obstetrician and anaesthetist specialised in managing high-risk pregnancies, and a neonatologist [5, 6]. The woman must be followed at least monthly. The cardiac demands should be minimised by rest and a low salt diet. To avoid caval vein compression, the patient should lie in the lateral position. Hospital admission in the second trimester is often advised, though its benefit is unproven and a satisfactory outcome of pregnancy has been described when women were followed on an outpatient basis [1, 5, 6, 11, 13]. Oxygen should be given when hypoxaemia is present. Anticoagulation therapy should be continued when there is an established indication outside pregnancy. It should, however, be considered on an individual basis in other women, since there may be bleeding risk, for example in women with Eisenmenger syndrome or portal hypertension. When therapeutic anticoagulation therapy is given, it is mandatory that the anticoagulation effect is monitored carefully and frequently, since dose requirements change during pregnancy with increase in plasma volume and glomerular filtration rate [1].
When heart failure occurs, diuretics are the appropriate therapy, preferably furosemide. Spironolactone is contraindicated because of anti-androgenic effects.
The ESC guidelines on the management of pregnant women with heart disease advise that women who are on drug therapy for pulmonary hypertension continue this therapy during pregnancy [1]. Since bosentan has teratogenic effects, patients should be informed about the foetal risks, and substitution with other targeted drugs is often advised [1, 6, 11]. It is probably beneficial to start advanced therapy with prostacyclin analogues or sildenafil early during pregnancy [6, 11].
A timely delivery plan should be constructed and be available to all team members [14]. The best mode of delivery is a matter of debate. Vaginal delivery is associated with volume changes during contractions which poses a problem in women with pulmonary hypertension since they have limited capability to increase their cardiac output. Moreover, pushing can have adverse haemodynamic effects. Planned caesarean delivery may therefore be a better choice, though vaginal delivery is not considered absolutely contraindicated [1]. In many cases vaginal delivery will not be an option because early delivery is often necessary. When caesarean section is performed, general anaesthesia has several disadvantages that should be taken into account. These include cardiodepression by volatile agents, and increase of pulmonary vascular resistance during intubation and positive pressure ventilation. A careful combined epidural and low-dose spinal anaesthesia avoids vasodilatation. It can be safely applied by an experienced anaesthetist and is probably the best option. During delivery haemodynamic monitoring of arterial and central venous pressure is advised. Pulmonary artery pressure monitoring probably has more risks than advantages [1, 5, 6, 11, 13]. Hospital observation is necessary until at least 2 weeks after delivery [13].
Conclusion
Pulmonary hypertension is associated with high mortality and morbidity risks for the mother. The risk is increased in all groups of pulmonary hypertension (1 to 5). Although women with severe pulmonary hypertension seem to be at higher risk, a safe cut-off value is not known. Pregnancy is therefore contraindicated, but if a woman chooses to continue pregnancy, multidisciplinary care in highly specialised services is mandatory. New targeted anti-pulmonary hypertension therapy may decrease the risk.
References
Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology. Eur Heart J. 2011;Aug 26. doi:10.1093/eurheartj/ehr218.
Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2009;30:2493–537.
Thorne S, McGregor A, Nelson-Piercy C, et al. Risk of contraception and pregnancy in heart disease. Heart. 2006;92:1520–5.
Pieper PG. Pre-pregnancy risk assessment and counseling of the cardiac patient. Neth Heart J 2011;19:477–481.
Madden BP. Pulmonary hypertension and pregnancy. Int J Obstet Anesth. 2009;18:156–64.
Hsu CH, Gomberg-Maitland M, Glassner C, et al. The management of pregnancy and pregnancy-related medical conditions in pulmonary arterial hypertension patients. Int J Clin Pract. 2011;65 Suppl 172:6–14.
Drenthen W, Boersma E, Balci A, et al. On behalf of the ZAHARA investigators. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31:2124–32.
Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104(5):515–21.
Weiss BM, Zemp L, Seifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic review from 1978 through 1996. J Am Coll Cardiol. 1998;31:1650–7.
Bédard E, Dimopulos K, Gatzoulis M, et al. Has there been any progress made on pregnancy outcomes among women with pulmonary hypertension? Eur Heart J. 2009;30:256–65.
Kiely DG, Condliffe R, Webster V, et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG. 2010;117:565–74.
Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. 2007;49:2303–11.
Warnes CA. Pregnancy and pulmonary hypertension. Int J Cardiol. 2004;97:11–3.
Pieper PG. The pregnant woman with heart disease: management of pregnancy and delivery. Neth Heart J. 2011;xxxxxxxxx.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pieper, P.G., Hoendermis, E.S. Pregnancy in women with pulmonary hypertension. Neth Heart J 19, 504–508 (2011). https://doi.org/10.1007/s12471-011-0219-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-011-0219-9