Key Points
-
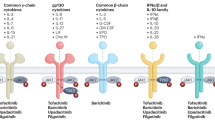
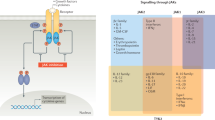
Despite differences in selectivity between Janus kinase (JAK) inhibitors, a large overlap exists in their safety profiles
-
All JAK inhibitors have been associated with a decrease in neutrophil number, although changes in numbers of lymphocytes and natural killer cells vary between compounds
-
An increased risk of viral infections (particularly herpes zoster) seems to distinguish the safety profile of tofacitinib from that of biologic DMARDs
-
Similarly to tofacitinib, other JAK inhibitors also seem to increase the risk of herpes zoster infection despite differences in JAK selectivity
-
To date, no increased risk of malignancy has been reported with tofacitinib in rheumatoid arthritis; however, experience is limited and this risk must be evaluated in the long term with all JAK inhibitors
-
The prevention of herpes zoster and other opportunistic infections is both feasible and important in the setting of JAK inhibition for the treatment of autoimmune inflammatory diseases
Abstract
Tofacitinib is the first Janus kinase (JAK) inhibitor commercially approved for the treatment of rheumatoid arthritis. This compound and a number of other JAK inhibitors are currently being tested in phase II and III trials for the treatment of a variety of autoimmune inflammatory diseases. Whereas a characteristic safety profile is emerging for some JAK inhibitors, differences between individual agents might emerge on the basis of distinct potency against their molecular targets. Similarly to biological therapy, JAK inhibition can lead to serious and opportunistic infections, and viral infections seem to be particularly frequent. Although no malignancy signals have been identified to date, long-term follow-up and further research are needed to understand the risk of malignancy associated with these compounds. As is the case for biologic agents, vaccination is important to mitigate the risks of these emerging therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
31 March 2017
In 'Gastrointestinal perforation' of the 'Adverse effects of JAK inhibitors' section, in the sentence “In patients with RA receiving baricitinib, two cases of gastrointestinal perforations were reported (an incidence of 5 cases per 1,000 patient-years in the development program)” the incidence should have been 0.5 cases per 1,000 patient-years. This error has been corrected in the online version of the article.
References
O'Shea, J. J., Pesu, M., Borie, D. C. & Changelian, P. S. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat. Rev. Drug Discov. 3, 555–564 (2004).
O'Shea, J. J., Laurence, A. & McInnes, I. B. Back to the future: oral targeted therapy for RA and other autoimmune diseases. Nat. Rev. Rheumatol. 9, 173–182 (2013).
Darnell, J. E. Jr, Kerr, I. M. & Stark, G. R. Jak–STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 (1994).
Leonard, W. J. & O'Shea, J. J. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16, 293–322 (1998).
O'Shea, J. J. & Murray, P. J. Cytokine signaling modules in inflammatory responses. Immunity 28, 477–487 (2008).
O'Shea, J. J. & Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36, 542–550 (2012).
Maertzdorf, J. et al. Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PLoS ONE 6, e26938 (2011).
Clark, J. D., Flanagan, M. E. & Telliez, J. B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 57, 5023–5038 (2014).
Thoma, G. et al. Identification of a potent Janus kinase 3 inhibitor with high selectivity within the Janus kinase family. J. Med. Chem. 54, 284–288 (2011).
Genovese, M. C. et al. Longterm safety and efficacy of tocilizumab in patients with rheumatoid arthritis: a cumulative analysis of up to 4.6 years of exposure. J. Rheumatol. 40, 768–780 (2013).
Genovese, M. C. et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 58, 2968–2980 (2008).
Gabay, C. et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381, 1541–1550 (2013).
Wollenhaupt, J. et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J. Rheumatol. 41, 837–852 (2014).
Wollenhaupt, J. et al. THU0185. Tofacitinib, an oral JAK inhibitor, in the treatment of rheumatoid arthritis: safety and clinical and radiographic efficacy in open-label, long-term extension studies over 7 years. Ann. Rheum. Dis. 75 (Suppl. 2), 252 (2016).
van Vollenhoven, R. et al. THU0178. Relationship between NK cell count and important safety events in rheumatoid arthritis patients treated with tofacitinib. Ann. Rheum. Dis. 74 (Suppl. 2), 258–259 (2015).
Kremer, J. M. et al. Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial. Arthritis Res. Ther. 17, 95 (2015).
Genovese, M. C. et al. OP0029. Baricitinib, an oral Janus kinase (JAK)1/JAK2 inhibitor, in patients with active rheumatoid arthritis (RA) and an inadequate response to TNF inhibitors: results of the phase 3 RA-Beacon study [abstract]. Ann. Rheum. Dis. 74 (Suppl. 2), 75–76 (2015).
Smolen, J. et al. THU0166. Safety profile of baricitinib in patients with active RA: an integrated analysis [abstract]. Ann. Rheum. Dis. 75 (Suppl. 2), 243–244 (2016).
Tanaka, Y. et al. THU0209. Characterisation of changes in lymphocyte subsets in baricitinib-treated patients with rheumatoid arthritis in a phase 3 study (RA-BEAM) [abstract]. Ann. Rheum. Dis. 75, 262–263 (2016).
Emery, P. et al. A7.16. Characterization of changes in lymphocyte subsets in baricitinib-treated patients with rheumatoid arthritis in two phase 3 studies [abstract]. Arthritis Rheumatol. 67 (Suppl. 10), 1047 (2016).
Dougados, M., et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann. Rheum. Dis. 76, 88–95 (2017).
Fleischmann, R. M. et al. A double-blind, placebo-controlled, twelve-week, dose-ranging study of decernotinib, an oral selective JAK-3 inhibitor, as monotherapy in patients with active rheumatoid arthritis. Arthritis Rheumatol. 67, 334–343 (2015).
Takeuchi, T. et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann. Rheum. Dis. 75, 1057–1064 (2015).
Kremer, J. M. et al. A phase 2b study of ABT-494, a selective JAK1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti–TNF therapy. Arthritis Rheumatol. 68, 2867–2877 (2016).
Genovese, M. C. et al. A randomized phase 2b study of ABT-494, a selective JAK1 inhibitor in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 68, 2857–2866 (2016).
Westhovens, R. et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2016-210104 (2016).
Kavanaugh, A. et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2016-210105 (2016).
Askling, J. et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol. Drug Saf. 20, 119–130 (2011).
Diamond, M. S. et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208, 1989–2003 (2011).
Curtis, J. R. et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann. Rheum. Dis. 75, 831–841 (2015).
Weinblatt, M. E. et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J. Rheumatol. 40, 787–797 (2013).
van Vollenhoven, R. F. et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann. Rheum. Dis. 72, 1496–1502 (2013).
Smolen, J. S. et al. Golimumab in patients with active rheumatoid arthritis who have previous experience with tumour necrosis factor inhibitors: results of a long-term extension of the randomised, double-blind, placebo-controlled GO-AFTER study through week 160. Ann. Rheum. Dis. 71, 1671–1679 (2012).
Simon, T. A. et al. Malignancies in the rheumatoid arthritis abatacept clinical development programme: an epidemiological assessment. Ann. Rheum. Dis. 68, 1819–1826 (2009).
Bykerk, V. P. et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann. Rheum. Dis. 74, 96–103 (2015).
Burmester, G. R., Panaccione, R., Gordon, K. B., McIlraith, M. J. & Lacerda, A. P. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann. Rheum. Dis. 72, 517–524 (2013).
Gottlieb, A. B. et al. Clinical trial safety and mortality analyses in patients receiving etanercept across approved indications. J. Drugs Dermatol. 10, 289–300 (2011).
Dougados, M. et al. LB0001. Baricitinib, an oral Janus kinase (JAK)1/JAK2 inhibitor, in patients with active rheumatoid arthritis (RA) and an inadequate response to cDMARD therapy: results of the phase 3 RA-build study [abstract]. Ann. Rheum. Dis. 74 (Suppl. 2), 79 (2015).
Cohen, S. et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 66, 2924–2937 (2014).
Doran, M. F., Crowson, C. S., Pond, G. R., O'Fallon, W. M. & Gabriel, S. E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 46, 2287–2293 (2002).
Harpaz, R., Ortega-Sanchez, I. R. & Seward, J. F. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly Rep. 57, 1–30 (2008).
Winthrop, K. L. et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA 309, 887–895 (2013).
Smitten, A. L. et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 57, 1431–1438 (2007).
Schmajuk, G. et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA 305, 480–486 (2011).
Winthrop, K. L. & Furst, D. E. Rheumatoid arthritis and herpes zoster: risk and prevention in those treated with anti-tumour necrosis factor therapy. Ann. Rheum. Dis. 69, 1735–1737 (2010).
Winthrop, K. L. et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 66, 2675–2684 (2014).
Winthrop, K. et al. SAT0229. Herpes zoster and tofacitinib: the risk of concomitant nonbiologic therapy. Ann. Rheum. Dis. 74, 741 (2015).
Curtis, J. R., Xie, F., Yun, H., Bernatsky, S. & Winthrop, K. L. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann. Rheum. Dis. 75, 1843–1847 (2016).
Winthrop, K. L. et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann. Rheum. Dis. 75, 1133–1138 (2016).
Genovese, M. C., van Vollenhoven, R., Bloom, B. J., Jiang, J. G. & Kinnman, N. A phase 2b, 12-week study of VX-509, an oral selective Janus kinase 3 inhibitor, in combination with background methotrexate in rheumatoid arthritis [abstract]. Arthritis Rheum. http://acrabstracts.org/abstract/a-phase-2b-12-week-study-of-vx-509-an-oral-selective-janus-kinase-3-inhibitor-in-combination-with-background-methotrexate-in-rheumatoid-arthritis/ (2013).
Genovese, M. C., van Vollenhoven, R. F., Pacheco-Tena, C., Zhang, Y. & Kinnman, N. VX-509 (decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. 68, 46–55 (2016).
Jung, C. W. et al. Efficacy and safety of ruxolitinib in Asian patients with myelofibrosis. Leuk. Lymphoma 56, 2067–2074 (2015).
Vannucchi, A. M. et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N. Engl. J. Med. 372, 426–435 (2015).
Khamashta, M. et al. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 1909–1916 (2016).
Winthrop, K. L. et al. Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann. Rheum. Dis. 74, 2107–2116 (2015).
Wathes, R., Moule, S. & Milojkovic, D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N. Engl. J. Med. 369, 197–198 (2013).
Chapgier, A. et al. A partial form of recessive STAT1 deficiency in humans. J. Clin. Invest. 119, 1502–1514 (2009).
Dupuis, S. et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33, 388–391 (2003).
O'Shea, J. J., Holland, S. M. & Staudt, L. M. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 368, 161–170 (2013).
Xeljanz® (tofacitinib citrate) package insert (Pfizer, 2012).
Genovese, M. C. et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 374, 1243–1252 (2016).
Cohen, S., Curtis, J. R., Fleischmann, R. & Chen, Y. 18-month worldwide post-marketing surveillance experience of tofacitinib [abstract 465]. Arthritis Rheum. 74 (Suppl.), S199 (2014).
Xie, F., Yun, H., Bernatsky, S. & Curtis, J. R. Risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 68, 2612–2617 (2016).
Marren, A., Chen, Y., Frazier, D. & Geier, J. THU0173. Pregnancy outcomes in the tofacitinib RA safety database through April 2014. Ann. Rheum. Dis. 74, 256–257 (2015).
Singh, J. A. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. (Hoboken) 68, 1–25 (2016).
Zhang, J. et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 308, 43–49 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02538757 (2017).
Rubin, L. G. et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 58, e44–e100 (2014).
Hales, C. M., Harpaz, R., Oretga-Sanchez, I. & Bialek, S. Update on recommendations for use of herpes zoster vaccine. MMWR Morbid. Mortal. Wkly Rep. 63, 729–731 (2014).
Pierson, D. L. et al. Varicella zoster virus DNA at inoculation sites and in saliva after Zostavax immunization. J. Infect. Dis. 203, 1542–1545 (2011).
Winthrop, K. et al. Assessment of immunogenicity of live zoster vaccination in rheumatoid arthritis patients on background methotrexate before and after initiating tofacitinib or placebo [abstract]. Arthritis Rheumatol. 67 (Suppl. 10), 12L (2016).
Oxman, M. N. et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352, 2271–2284 (2005).
Morrison, V. A. et al. Long-term persistence of zoster vaccine efficacy. Clin. Infect. Dis. 60, 900–909 (2015).
Lal, H. et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 372, 2087–2096 (2015).
Acknowledgements
The author thanks M. Morgove for assistance with formatting and references.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares that he has received research support from and has acted as a consultant for Abbvie, Astellis, Galapagos, Lilly, Pfizer, BMS and UCB.
Rights and permissions
About this article
Cite this article
Winthrop, K. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 13, 234–243 (2017). https://doi.org/10.1038/nrrheum.2017.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.23
This article is cited by
-
Janus kinase inhibitors vs. abatacept about safety and efficacy for patients with rheumatoid arthritis-associated interstitial lung disease: a retrospective nested case-control study
BMC Rheumatology (2024)
-
Efficacy and safety of JAK inhibitors in rheumatoid arthritis: update for the practising clinician
Nature Reviews Rheumatology (2024)
-
Clinical Pharmacokinetic and Pharmacodynamic Considerations in the Treatment of Moderate-to-Severe Psoriasis
Clinical Pharmacokinetics (2024)
-
Clinical prediction models of rheumatoid arthritis and its complications: focus on cardiovascular disease and interstitial lung disease
Arthritis Research & Therapy (2023)
-
Efficacy and safety of baricitinib in treatment of systemic lupus erythematosus: a systematic review and meta-analysis
BMC Rheumatology (2023)