-
PDF
- Split View
-
Views
-
Cite
Cite
Vimalanand S Prabhu, Erik R Dubberke, Mary Beth Dorr, Elamin Elbasha, Nicole Cossrow, Yiling Jiang, Stephen Marcella, Cost-effectiveness of Bezlotoxumab Compared With Placebo for the Prevention of Recurrent Clostridium difficile Infection, Clinical Infectious Diseases, Volume 66, Issue 3, 1 February 2018, Pages 355–362, https://doi.org/10.1093/cid/cix809
Close - Share Icon Share
Abstract
Clostridium difficile infection (CDI) is the most commonly recognized cause of recurrent diarrhea. Bezlotoxumab, administered concurrently with antibiotics directed against C. difficile (standard of care [SoC]), has been shown to reduce the recurrence of CDI, compared with SoC alone. This study aimed to assess the cost-effectiveness of bezlotoxumab administered concurrently with SoC, compared with SoC alone, in subgroups of patients at risk of recurrence of CDI.
A computer-based Markov health state transition model was designed to track the natural history of patients infected with CDI. A cohort of patients entered the model with either a mild/moderate or severe CDI episode, and were treated with SoC antibiotics together with either bezlotoxumab or placebo. The cohort was followed over a lifetime horizon, and costs and utilities for the various health states were used to estimate incremental cost-effectiveness ratios (ICERs). Both deterministic and probabilistic sensitivity analyses were used to test the robustness of the results.
The cost-effectiveness model showed that, compared with placebo, bezlotoxumab was associated with 0.12 quality-adjusted life-years (QALYs) gained and was cost-effective in preventing CDI recurrences in the entire trial population, with an ICER of $19824/QALY gained. Compared with placebo, bezlotoxumab was also cost-effective in the subgroups of patients aged ≥65 years (ICER of $15298/QALY), immunocompromised patients (ICER of $12597/QALY), and patients with severe CDI (ICER of $21430/QALY).
Model-based results demonstrated that bezlotoxumab was cost-effective in the prevention of recurrent CDI compared with placebo, among patients receiving SoC antibiotics for treatment of CDI.
Clostridium difficile infection (CDI) is the most commonly recognized cause of diarrhea-associated nosocomial infection in adults in the United States and Europe [1, 2]. In adults, the spectrum of CDI includes abdominal pain, profuse watery diarrhea, pseudomembranous colitis, and death. A common feature of CDI is its frequent recurrence, either as a relapse or reinfection after an initial resolution of symptoms following antibiotic therapy. Around 25% of patients experience a recurrent infection within 30 days of completing antibiotic therapy, and the likelihood of recurrence increases with each subsequent CDI episode. Of those who have a primary recurrence, approximately 35% [3] will have a further CDI episode, and after 2 recurrences, the likelihood of an additional episode increases to as much as 45%–65% [4].
Risk factors for the recurrence of CDI infection include advanced age (>65 years), a weakened immune system, infection with C. difficile ribotype 027, exposure to antibiotics, hospitalization/length of hospital stay, comorbidities (such as inflammatory bowel disease, colorectal cancer, or kidney disease), use of proton pump inhibitors, and surgery of the gastrointestinal tract [5–9].
The current health burden and management costs of CDI are substantial. The aggregated data for the United States in 2009 showed that the all-cause cost of all CDI hospital stays reached $8.2 billion, which is 2.3% of total US hospital costs [10, 11]. Patients with recurrent CDI are 12.5 times more likely to have inpatient hospital costs than patients who do not develop recurrences (P = .07) [12]. This is primarily due to the increase in readmissions. In a matched cohort study in the United States using medical record data from 2011, the attributable cost of a recurrent CDI episode was estimated to be $11146 over and above that of an initial CDI episode only ($8448 for an initial episode) [12].
A significant global economic burden is therefore associated with recurrent CDI, as it is more difficult to treat than initial episodes and is associated with increased morbidity and mortality [1, 13], more hospitalizations, and higher associated costs [4].
Recently, bezlotoxumab, a fully human monoclonal antibody directed against the toxin B produced by C. difficile, has been introduced as a new approach to the prevention of CDI recurrence in patients receiving antibiotic therapy for CDI.
Bezlotoxumab prevents recurrence by a different mechanism of action. It binds with C. difficile toxin B and neutralizes toxin activity by preventing it from binding to host cells. It is thought that neutralization of toxin after completion of a course of effective antibiotic treatment prevents new CDI symptoms in the setting of C. difficile regrowth. Restoration of a healthy gut microbiota, the body’s natural defense against C. difficile, can occur naturally so the need for additional antibiotic treatment is avoided and relapse or reinfection is prevented.
Two randomized, double-blind, placebo-controlled, global phase 3 trials (monoclonal antibodies for C. difficile therapy [MODIFY] and MODIFY II) were conducted between 2011 and 2015 to evaluate the efficacy, safety, and tolerability of bezlotoxumab in 2655 adult patients with CDI who were receiving standard of care (SoC) antibiotic therapy for a primary or recurrent episode of CDI [14]. The primary endpoint in both trials was CDI recurrence following clinical cure of the baseline CDI episode during 12 weeks of follow-up.
Efficacy data from the pooled trials demonstrated that bezlotoxumab was associated with a statistically significant and clinically meaningful 38% relative reduction in CDI recurrence compared with placebo when used together with SoC antibiotics for the treatment of CDI. The SoC antibiotics used in the trials included metronidazole, vancomycin, and fidaxomicin.
Bezlotoxumab has now been approved in the United States and the European Union for the prevention of recurrent CDI in patients at high risk of CDI recurrence [15, 16]. Given the significant clinical and economic burden of recurrent CDI, and the ability of bezlotoxumab to reduce recurrent CDI, it is important to assess the cost-effectiveness of bezlotoxumab compared with placebo in preventing CDI among various subgroups of patients receiving SoC antibiotics.
Therefore, the objective of our study was to evaluate the cost-effectiveness of bezlotoxumab compared with placebo from a third-party payer perspective.
METHODS
Patient Population
The cost-effectiveness of bezlotoxumab will likely vary in different subgroups because of differences in the underlying risk of recurrence, the efficacy of bezlotoxumab in reducing a recurrence, CDI-attributable mortality, and the cost of a CDI recurrence. This study population was based on the pooled modified intention-to-treat population from the MODIFY I/II clinical trials, which included all randomly assigned participants who received the study infusion, had a baseline stool test that was positive for toxigenic C. difficile, and began SoC antibiotic therapy prior to or within 1 day after receiving bezlotoxumab [14].
To obtain a comprehensive picture of the economic costs and benefits, as well as modeling the entire MODIFY I/II trial population (base case), we assessed the cost-effectiveness in the following subpopulations that were prespecified in the MODIFY trials: patients aged ≥65 years at the time of infusion, patients who were immunocompromised (based on underlying disease or immunosuppressive therapy) at the time of infusion, and patients with a clinically severe CDI episode (based on Zar score ≥2) at the time of infusion.
These 3 subgroups were further stratified into those having 1 or more episodes of CDI within the previous 6 months, thereby creating a total of 6 subgroups. These combined subgroups were considered to have the greatest baseline risk of CDI recurrence.
Analytic Framework
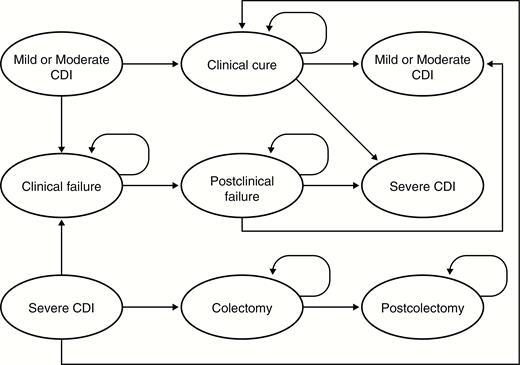
A computer-based Markov health state transition model (NO-RCDI) was built in Microsoft Excel (Microsoft Corporation, Redmond, Washington) to simulate the natural history of recurrent CDI (Figure 1). The model follows the natural history of CDI in a cohort of patients infected with C. difficile from infection until death. The patients can enter the model with an initial episode of CDI or a recurrent episode; this CDI episode can be mild/moderate or severe. Patients with mild/moderate CDI are treated with SoC antibiotics, after which they can either be cured (clinical cure health state) or experience clinical failure (clinical failure health state). Clinical cure and clinical failure are defined as in the MODIFY I/II trials [14]. The duration of antibiotic therapy was taken into account and was based on the suggested duration for metronidazole and vancomycin in the Society for Healthcare Epidemiology of America/Infectious Diseases Society of America treatment guidelines for C. difficile [17]. Patients experienced clinical cure when they received ≤14 days’ regimen of SoC therapy and had no diarrhea (≤2 loose stools per 24 hours) for 2 consecutive days following completion of SoC therapy for the baseline CDI episode. Those experiencing clinical failure required >14 days’ regimen of SoC therapy for the baseline CDI episode or had ≥3 loose stools on 1 or both of the 2 consecutive days following completion of SoC therapy for the baseline CDI episode.
Markov health state transition model structure. The model simulates the natural history of recurrent Clostridium difficile infection (CDI) from infection to death. Patients can enter the model with an initial or recurrent episode of CDI (episodes can be mild/moderate or severe). Patients with mild/moderate CDI are treated with standard-of-care antibiotics, after which they can either be cured (clinical cure health state) or experience clinical failure (clinical failure health state). Patients with severe CDI may follow a similar history to those with mild/moderate CDI or may require a colectomy; patients in the postcolectomy health state cannot have a recurrence as CDI symptoms are generally characterized by colitis. A person can experience death in any health state.
Abbreviation: CDI, Clostridium difficile infection.
A patient with clinical failure is treated further and may eventually be cured (postclinical failure health state). In the current analysis, it was assumed that patients in postclinical failure health state would not experience any recurrence of CDI, as CDI recurrence in the trial was only assessed in those who had a clinical cure. Moreover, it was assumed that the consequences of these patients would not differ between treatment arms. Once patients are cured, they can experience a CDI recurrence (mild/moderate or severe).
Patients who enter the model with severe CDI follow a similar natural history to patients with mild/moderate CDI, except that those with severe CDI may require a colectomy. Patients who have had a colectomy move to a postcolectomy health state and can no longer have a recurrence, as CDI symptoms are generally characterized by the presence of colitis.
Patients can die in any health state. The model allows for a different mortality rate in the first 6 months, to account for the increased risk of CDI-attributable mortality [13]. Because CDI is a recurrent disease, the natural history is repeated for each recurrence, and a patient can have up to 3 subsequent recurrences postinfusion in the model.
Thus, a patient treated for a second recurrence (third overall CDI episode) is followed until the fifth recurrence. Patients who remain permanently in the “clinical cure” health state, without experiencing subsequent recurrence or death, are considered to have achieved sustained response. The natural history is identical for both the treatment and comparator arms. The rate of recurrence in the treatment arm can vary depending upon the clinical efficacy of bezlotoxumab.
Comparators, Scope, Time Horizon, and Perspective
The model compares the clinical and economic benefits of bezlotoxumab administered in addition to SoC, compared with placebo. SoC antibiotics can include oral or intravenous metronidazole, oral vancomycin, or oral fidaxomicin. Bezlotoxumab was assumed to be efficacious for 12 weeks, which is the duration of the follow-up period in the MODIFY I/II clinical trials. Costs were assessed from a third-party payer perspective, and both costs and outcomes were discounted at 3% in accordance with published guidelines [18].
Because the benefits and costs of therapy extend throughout the patient’s life, a lifetime horizon was adopted for the analysis [19]. The lifetime horizon was divided into 2 parts: an initial 6 months with a cycle length of 15 days, and remaining life with an annual cycle length. A shorter cycle length was essential for the first 6 months as, during the early symptomatic phase after CDI infection, the recommended course of antibiotic therapy is 10–14 days [17], and the reduced cycle length enables us to model for the health state transition (such as from CDI to clinical cure or clinical failure) seamlessly. The 6-month initial horizon also enables us to account for the 6-monthly CDI-attributable mortality and CDI-attributable costs [12, 20].
Input Parameters
Clinical Inputs
The pooled data from the MODIFY I/II clinical trials (Table 1) show that a single dose of bezlotoxumab 10 mg/kg administered as an intravenous infusion was superior to placebo in the prevention of CDI recurrence through 12 weeks of follow-up (primary endpoint). The rate of first recurrence after infusion in the entire population was 26.6% and 16.5% in the placebo and treatment arms, respectively [14], while a recurrence rate of 45% was used for subsequent recurrences, based on a review of CDI literature [21], with 9.9% of recurrences considered to be severe [Ad hoc analysis of MODIFY I and MODIFY II clinical trials; unpublished data on file. Merck & Co, Inc. 2016].
Background Recurrence and Efficacy of Bezlotoxumab in Patient Subgroups Based on Pooled Data From the Clinical Trials
| Patient Subgroup . | Mean Age, y . | Proportion of Females, % . | Proportion of Patients Entering the Model With Severe CDI, % . | Recurrence on Bezlotoxumab, % . | Recurrence on Placebo, % . |
|---|---|---|---|---|---|
| Entire clinical trial population (base case) | 62.7 | 57.3 | 16.6 | 16.5 | 26.6 |
| Patients aged ≥65 y | 76.6 | 57.9 | 25.4 | 15.4 | 31.4 |
| Patients who are immunocompromised | 60.7 | 49.2 | 18.8 | 14.6 | 27.5 |
| Patients with severe CDI on presentation | 71.0 | 53.4 | 100.0 | 10.7 | 22.4 |
| Patients aged ≥65 y and ≥1 previous episode in prior 6 mo | 77.0 | 55.5 | 17.6 | 19.4 | 43.4 |
| Patients who are immunocompromised and ≥1 previous episode in prior 6 mo | 64.9 | 44.0 | 17.4 | 20.5 | 40.4 |
| Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo | 73.7 | 50.0 | 100.0 | 9.1 | 33.3 |
| Patient Subgroup . | Mean Age, y . | Proportion of Females, % . | Proportion of Patients Entering the Model With Severe CDI, % . | Recurrence on Bezlotoxumab, % . | Recurrence on Placebo, % . |
|---|---|---|---|---|---|
| Entire clinical trial population (base case) | 62.7 | 57.3 | 16.6 | 16.5 | 26.6 |
| Patients aged ≥65 y | 76.6 | 57.9 | 25.4 | 15.4 | 31.4 |
| Patients who are immunocompromised | 60.7 | 49.2 | 18.8 | 14.6 | 27.5 |
| Patients with severe CDI on presentation | 71.0 | 53.4 | 100.0 | 10.7 | 22.4 |
| Patients aged ≥65 y and ≥1 previous episode in prior 6 mo | 77.0 | 55.5 | 17.6 | 19.4 | 43.4 |
| Patients who are immunocompromised and ≥1 previous episode in prior 6 mo | 64.9 | 44.0 | 17.4 | 20.5 | 40.4 |
| Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo | 73.7 | 50.0 | 100.0 | 9.1 | 33.3 |
Source: Wilcox et al [14].
Abbreviation: CDI, Clostridium difficile infection.
Background Recurrence and Efficacy of Bezlotoxumab in Patient Subgroups Based on Pooled Data From the Clinical Trials
| Patient Subgroup . | Mean Age, y . | Proportion of Females, % . | Proportion of Patients Entering the Model With Severe CDI, % . | Recurrence on Bezlotoxumab, % . | Recurrence on Placebo, % . |
|---|---|---|---|---|---|
| Entire clinical trial population (base case) | 62.7 | 57.3 | 16.6 | 16.5 | 26.6 |
| Patients aged ≥65 y | 76.6 | 57.9 | 25.4 | 15.4 | 31.4 |
| Patients who are immunocompromised | 60.7 | 49.2 | 18.8 | 14.6 | 27.5 |
| Patients with severe CDI on presentation | 71.0 | 53.4 | 100.0 | 10.7 | 22.4 |
| Patients aged ≥65 y and ≥1 previous episode in prior 6 mo | 77.0 | 55.5 | 17.6 | 19.4 | 43.4 |
| Patients who are immunocompromised and ≥1 previous episode in prior 6 mo | 64.9 | 44.0 | 17.4 | 20.5 | 40.4 |
| Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo | 73.7 | 50.0 | 100.0 | 9.1 | 33.3 |
| Patient Subgroup . | Mean Age, y . | Proportion of Females, % . | Proportion of Patients Entering the Model With Severe CDI, % . | Recurrence on Bezlotoxumab, % . | Recurrence on Placebo, % . |
|---|---|---|---|---|---|
| Entire clinical trial population (base case) | 62.7 | 57.3 | 16.6 | 16.5 | 26.6 |
| Patients aged ≥65 y | 76.6 | 57.9 | 25.4 | 15.4 | 31.4 |
| Patients who are immunocompromised | 60.7 | 49.2 | 18.8 | 14.6 | 27.5 |
| Patients with severe CDI on presentation | 71.0 | 53.4 | 100.0 | 10.7 | 22.4 |
| Patients aged ≥65 y and ≥1 previous episode in prior 6 mo | 77.0 | 55.5 | 17.6 | 19.4 | 43.4 |
| Patients who are immunocompromised and ≥1 previous episode in prior 6 mo | 64.9 | 44.0 | 17.4 | 20.5 | 40.4 |
| Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo | 73.7 | 50.0 | 100.0 | 9.1 | 33.3 |
Source: Wilcox et al [14].
Abbreviation: CDI, Clostridium difficile infection.
Clinical cure rates from MODIFY I/II [Ad hoc analysis of MODIFY I and MODIFY II clinical trials; unpublished data on file. Merck & Co, Inc. 2016] were used to populate the efficacy of SoC (80.9% for index case and 78.7% for first recurrence) in the treatment of CDI, with a risk of colectomy assumed to be 0.7%, and the risk of death among patients requiring colectomy estimated to be 30.7% (Table 2) [22].
Clinical Input Parameters
| Parameter . | Value . | Source . |
|---|---|---|
| 30-d probability of first recurrence after infusion | Various as per Table 3 | Clinical trial data |
| 30-d probability of second and later recurrences after infusion | 45% | Kelly, 2012 [21] |
| 180-d all-cause mortality following CDI | 25.7% in those without a subsequent recurrence, 36.3% in those with 1 or more subsequent recurrences | Olsen et al, 2015 [20] |
| CDI-attributable cost of a recurrence | $13386 | Dubberke et al, 2014, indexed using medical care component of CPI (2015) [12] |
| Proportion of recurrences that are severe | 9.9% | Data on file, 2016 |
| Probability of colectomy | 0.7% | Halabi et al, 2013 [22] |
| Probability of death after colectomy | 30.7% | Halabi et al, 2013 [22] |
| QALY – utility weight for mild CDI | 0.880 | Bartsch et al, 2013 [25] |
| QALY – utility weight for severe CDI | 0.817 | Bartsch et al, 2013 [25] |
| QALY – utility weight for colectomy | 0.817 | Bartsch et al, 2013 [25] |
| QALY – utility weight for clinical cure, clinical failure, postcolectomy | 1.00 | Bartsch et al, 2013 [25] |
| SoC assumptions | Assumed to be consistent with MODIFY I and II | Wilcox et al, 2017 [14] |
| SoC efficacy for index case | 80.9% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| SoC efficacy for first recurrence | 78.7% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| SoC efficacy for second and third recurrence | 81.4% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| Duration of CDI episode | 15 d | |
| Duration of clinical response | 15 d | |
| Duration of clinical failure | Additional 15 d | |
| Duration of bezlotoxumab efficacy | 84 d | |
| Duration of colectomy episode | 15 d |
| Parameter . | Value . | Source . |
|---|---|---|
| 30-d probability of first recurrence after infusion | Various as per Table 3 | Clinical trial data |
| 30-d probability of second and later recurrences after infusion | 45% | Kelly, 2012 [21] |
| 180-d all-cause mortality following CDI | 25.7% in those without a subsequent recurrence, 36.3% in those with 1 or more subsequent recurrences | Olsen et al, 2015 [20] |
| CDI-attributable cost of a recurrence | $13386 | Dubberke et al, 2014, indexed using medical care component of CPI (2015) [12] |
| Proportion of recurrences that are severe | 9.9% | Data on file, 2016 |
| Probability of colectomy | 0.7% | Halabi et al, 2013 [22] |
| Probability of death after colectomy | 30.7% | Halabi et al, 2013 [22] |
| QALY – utility weight for mild CDI | 0.880 | Bartsch et al, 2013 [25] |
| QALY – utility weight for severe CDI | 0.817 | Bartsch et al, 2013 [25] |
| QALY – utility weight for colectomy | 0.817 | Bartsch et al, 2013 [25] |
| QALY – utility weight for clinical cure, clinical failure, postcolectomy | 1.00 | Bartsch et al, 2013 [25] |
| SoC assumptions | Assumed to be consistent with MODIFY I and II | Wilcox et al, 2017 [14] |
| SoC efficacy for index case | 80.9% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| SoC efficacy for first recurrence | 78.7% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| SoC efficacy for second and third recurrence | 81.4% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| Duration of CDI episode | 15 d | |
| Duration of clinical response | 15 d | |
| Duration of clinical failure | Additional 15 d | |
| Duration of bezlotoxumab efficacy | 84 d | |
| Duration of colectomy episode | 15 d |
Abbreviations: CDI, Clostridium difficile infection; CPI, Consumer Price Index; MODIFY, monoclonal antibodies for C. difficile therapy; QALY, quality-adjusted life-year; SoC, standard of care.
Clinical Input Parameters
| Parameter . | Value . | Source . |
|---|---|---|
| 30-d probability of first recurrence after infusion | Various as per Table 3 | Clinical trial data |
| 30-d probability of second and later recurrences after infusion | 45% | Kelly, 2012 [21] |
| 180-d all-cause mortality following CDI | 25.7% in those without a subsequent recurrence, 36.3% in those with 1 or more subsequent recurrences | Olsen et al, 2015 [20] |
| CDI-attributable cost of a recurrence | $13386 | Dubberke et al, 2014, indexed using medical care component of CPI (2015) [12] |
| Proportion of recurrences that are severe | 9.9% | Data on file, 2016 |
| Probability of colectomy | 0.7% | Halabi et al, 2013 [22] |
| Probability of death after colectomy | 30.7% | Halabi et al, 2013 [22] |
| QALY – utility weight for mild CDI | 0.880 | Bartsch et al, 2013 [25] |
| QALY – utility weight for severe CDI | 0.817 | Bartsch et al, 2013 [25] |
| QALY – utility weight for colectomy | 0.817 | Bartsch et al, 2013 [25] |
| QALY – utility weight for clinical cure, clinical failure, postcolectomy | 1.00 | Bartsch et al, 2013 [25] |
| SoC assumptions | Assumed to be consistent with MODIFY I and II | Wilcox et al, 2017 [14] |
| SoC efficacy for index case | 80.9% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| SoC efficacy for first recurrence | 78.7% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| SoC efficacy for second and third recurrence | 81.4% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| Duration of CDI episode | 15 d | |
| Duration of clinical response | 15 d | |
| Duration of clinical failure | Additional 15 d | |
| Duration of bezlotoxumab efficacy | 84 d | |
| Duration of colectomy episode | 15 d |
| Parameter . | Value . | Source . |
|---|---|---|
| 30-d probability of first recurrence after infusion | Various as per Table 3 | Clinical trial data |
| 30-d probability of second and later recurrences after infusion | 45% | Kelly, 2012 [21] |
| 180-d all-cause mortality following CDI | 25.7% in those without a subsequent recurrence, 36.3% in those with 1 or more subsequent recurrences | Olsen et al, 2015 [20] |
| CDI-attributable cost of a recurrence | $13386 | Dubberke et al, 2014, indexed using medical care component of CPI (2015) [12] |
| Proportion of recurrences that are severe | 9.9% | Data on file, 2016 |
| Probability of colectomy | 0.7% | Halabi et al, 2013 [22] |
| Probability of death after colectomy | 30.7% | Halabi et al, 2013 [22] |
| QALY – utility weight for mild CDI | 0.880 | Bartsch et al, 2013 [25] |
| QALY – utility weight for severe CDI | 0.817 | Bartsch et al, 2013 [25] |
| QALY – utility weight for colectomy | 0.817 | Bartsch et al, 2013 [25] |
| QALY – utility weight for clinical cure, clinical failure, postcolectomy | 1.00 | Bartsch et al, 2013 [25] |
| SoC assumptions | Assumed to be consistent with MODIFY I and II | Wilcox et al, 2017 [14] |
| SoC efficacy for index case | 80.9% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| SoC efficacy for first recurrence | 78.7% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| SoC efficacy for second and third recurrence | 81.4% | Clinical study reports of MODIFY I and II trials (bezlotoxumab and placebo arms) |
| Duration of CDI episode | 15 d | |
| Duration of clinical response | 15 d | |
| Duration of clinical failure | Additional 15 d | |
| Duration of bezlotoxumab efficacy | 84 d | |
| Duration of colectomy episode | 15 d |
Abbreviations: CDI, Clostridium difficile infection; CPI, Consumer Price Index; MODIFY, monoclonal antibodies for C. difficile therapy; QALY, quality-adjusted life-year; SoC, standard of care.
A mortality reduction was not attributed directly to bezlotoxumab, but rather a differential rate of mortality was applied to different health states. The risk of mortality during the first 180 days after infection was assumed to be 25.7% for patients with a sustained response, compared with 36.3% for those with a recurrence [20], whereas at 6 months postinfusion, the mortality risk was assumed to be the same for patients with or without a sustained response and was estimated from US life tables [23].
Cost Inputs
Patients with a recurrence were assumed to be more likely to have higher CDI-attributable costs than those who have a sustained response. Rather than attempting to calculate the costs of each recurrence, the methodology of Dubberke et al was used to estimate the total attributable cost over a 6-month period [12]. The estimates from Dubberke et al were also used to estimate the average cost of all patients in a cohort who had at least 1 recurrence in a 6-month period compared with a matched cohort that had no posttreatment recurrence, to estimate the CDI recurrence-attributable costs. While the model computes subsequent recurrences, the 6-month CDI recurrence-attributable cost (Table 2) was only applied to the first recurrence and not to subsequent recurrences to avoid double counting. The 6-month attributable cost is, in effect, a weighted average of all patients with at least a first recurrence and includes the whole spectrum of patients with recurrence, some of whom have >1 recurrence. Costs were inflated to 2015 US dollars adjusted for the US City Average Medical Care Consumer Price Index [24].
Utilities
The utility estimates for individual health states (Table 2) were based on those described by Bartsch et al [25]. Quality-adjusted life-years (QALYs) and cost-effectiveness ratios are all estimated by the model.
Model Analysis
The base case analysis includes the full trial population from MODIFY I/II (Table 1); outcomes include deaths, number of recurrences, number of colectomies, incremental number of recurrences, utilities, and costs. Deterministic (1-way) sensitivity analysis was used to test for uncertainty in variables such as duration of treatment effect, time horizon, and number of recurrences. Probabilistic sensitivity analysis, using Monte Carlo simulations methods to draw 1000 random samples from predefined distributions, was used to construct a cost-effectiveness acceptability curve for the entire population. This curve summarizes the uncertainty in the results of the cost-effectiveness analysis by depicting the probability that a regimen is cost-effective as a function of willingness to pay for a QALY gained [26]. The analysis was repeated for the other scenarios of high-risk patient subgroups.
RESULTS
In a cohort of patients with the same characteristics as those in the MODIFY I/II trials, the model predicted that, among patients receiving SoC, compared with placebo, the addition of bezlotoxumab can reduce the first recurrence by 10.1%, the total recurrences by 16.7%, and 180-day mortality by 1.1% (Table 3). The discounted incremental costs were estimated at $2444 per patient receiving bezlotoxumab, while the total number of QALYs was estimated to increase by 0.12 per patient treated, resulting in an incremental cost-effectiveness ratio (ICER) of $19824/QALY gained.
Cost-effectiveness of Bezlotoxumab + Standard of Care (SoC) Compared With Placebo + SoC
| Variable . | Placebo . | Bezlotoxumab . | Difference . |
|---|---|---|---|
| First recurrence | 26.6% | 16.5% | –10.1% |
| Second recurrence | 12.0% | 7.4% | –4.6% |
| Third recurrence | 5.4% | 3.3% | –2.1% |
| Total recurrence | 44.0% | 27.3% | –16.7% |
| NNT | 6.0 | 6.0 | |
| 180-d mortality | 28.5% | 27.5% | –1.1% |
| Undiscounted life-years | 14.59 | 14.81 | 0.22 |
| Discounted costs | $3567 | $6011 | $2444 |
| Discounted QALYs | 8.33 | 8.45 | 0.12 |
| ICER | $19824 | ||
| Variable . | Placebo . | Bezlotoxumab . | Difference . |
|---|---|---|---|
| First recurrence | 26.6% | 16.5% | –10.1% |
| Second recurrence | 12.0% | 7.4% | –4.6% |
| Third recurrence | 5.4% | 3.3% | –2.1% |
| Total recurrence | 44.0% | 27.3% | –16.7% |
| NNT | 6.0 | 6.0 | |
| 180-d mortality | 28.5% | 27.5% | –1.1% |
| Undiscounted life-years | 14.59 | 14.81 | 0.22 |
| Discounted costs | $3567 | $6011 | $2444 |
| Discounted QALYs | 8.33 | 8.45 | 0.12 |
| ICER | $19824 | ||
Abbreviations: ICER, incremental cost-effectiveness ratio; NNT, number needed to treat; QALY, quality-adjusted life-year.
Cost-effectiveness of Bezlotoxumab + Standard of Care (SoC) Compared With Placebo + SoC
| Variable . | Placebo . | Bezlotoxumab . | Difference . |
|---|---|---|---|
| First recurrence | 26.6% | 16.5% | –10.1% |
| Second recurrence | 12.0% | 7.4% | –4.6% |
| Third recurrence | 5.4% | 3.3% | –2.1% |
| Total recurrence | 44.0% | 27.3% | –16.7% |
| NNT | 6.0 | 6.0 | |
| 180-d mortality | 28.5% | 27.5% | –1.1% |
| Undiscounted life-years | 14.59 | 14.81 | 0.22 |
| Discounted costs | $3567 | $6011 | $2444 |
| Discounted QALYs | 8.33 | 8.45 | 0.12 |
| ICER | $19824 | ||
| Variable . | Placebo . | Bezlotoxumab . | Difference . |
|---|---|---|---|
| First recurrence | 26.6% | 16.5% | –10.1% |
| Second recurrence | 12.0% | 7.4% | –4.6% |
| Third recurrence | 5.4% | 3.3% | –2.1% |
| Total recurrence | 44.0% | 27.3% | –16.7% |
| NNT | 6.0 | 6.0 | |
| 180-d mortality | 28.5% | 27.5% | –1.1% |
| Undiscounted life-years | 14.59 | 14.81 | 0.22 |
| Discounted costs | $3567 | $6011 | $2444 |
| Discounted QALYs | 8.33 | 8.45 | 0.12 |
| ICER | $19824 | ||
Abbreviations: ICER, incremental cost-effectiveness ratio; NNT, number needed to treat; QALY, quality-adjusted life-year.
The ICERs for patients aged ≥65 years, who were immunocompromised, or with severe CDI on presentation, were $15298/QALY, $12597/QALY, and $21430/QALY, respectively (Table 4; Supplementary Tables A1–3). The ICERs for the further stratified subgroups of patients who had 1 or more episodes of CDI in the previous 6 months and who were aged ≥65 years, immunocompromised, or who had severe CDI on presentation were $3591/QALY, $4979/QALY, and $2938/QALY, respectively (Table 4; Supplementary Tables A4–6).
Cost-effectiveness of Bezlotoxumab + Standard of Care (SoC) Compared With Placebo + SoC in Various Subgroups
| Subgroup . | Incremental Total Recurrence (Bezlotoxumab – Placebo) . | NNT to Prevent a Recurrence . | Incremental Mortality (Bezlotoxumab – Placebo) . | Incremental Costs, $ (Bezlotoxumab – Placebo) . | Incremental QALYs (Bezlotoxumab – Placebo) . | ICER, $/QALY . |
|---|---|---|---|---|---|---|
| Patients aged ≥65 y | –26.4% | 3.8 | –1.7% | 1662 | 0.11 | 15298 |
| Patients who are immunocompromised | –21.2% | 4.7 | –1.4% | 2081 | 0.17 | 12597 |
| Patients with severe CDI on presentation | –19.5% | 5.1 | –1.2% | 2228 | 0.10 | 21430 |
| Patients aged ≥65 y and ≥1 previous episode in prior 6 mo | –39.7% | 2.5 | –2.6% | 587 | 0.16 | 3591 |
| Patients who are immunocompromised and ≥1 previous episode in prior 6 mo | –33.0% | 3.0 | –2.1% | 1127 | 0.23 | 4979 |
| Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo | –40.2% | 2.5 | –2.5% | 555 | 0.19 | 2938 |
| Subgroup . | Incremental Total Recurrence (Bezlotoxumab – Placebo) . | NNT to Prevent a Recurrence . | Incremental Mortality (Bezlotoxumab – Placebo) . | Incremental Costs, $ (Bezlotoxumab – Placebo) . | Incremental QALYs (Bezlotoxumab – Placebo) . | ICER, $/QALY . |
|---|---|---|---|---|---|---|
| Patients aged ≥65 y | –26.4% | 3.8 | –1.7% | 1662 | 0.11 | 15298 |
| Patients who are immunocompromised | –21.2% | 4.7 | –1.4% | 2081 | 0.17 | 12597 |
| Patients with severe CDI on presentation | –19.5% | 5.1 | –1.2% | 2228 | 0.10 | 21430 |
| Patients aged ≥65 y and ≥1 previous episode in prior 6 mo | –39.7% | 2.5 | –2.6% | 587 | 0.16 | 3591 |
| Patients who are immunocompromised and ≥1 previous episode in prior 6 mo | –33.0% | 3.0 | –2.1% | 1127 | 0.23 | 4979 |
| Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo | –40.2% | 2.5 | –2.5% | 555 | 0.19 | 2938 |
Abbreviations: CDI, Clostridium difficile infection; ICER, incremental cost-effectiveness ratio; NNT, number needed to treat; QALY, quality-adjusted life-year.
Cost-effectiveness of Bezlotoxumab + Standard of Care (SoC) Compared With Placebo + SoC in Various Subgroups
| Subgroup . | Incremental Total Recurrence (Bezlotoxumab – Placebo) . | NNT to Prevent a Recurrence . | Incremental Mortality (Bezlotoxumab – Placebo) . | Incremental Costs, $ (Bezlotoxumab – Placebo) . | Incremental QALYs (Bezlotoxumab – Placebo) . | ICER, $/QALY . |
|---|---|---|---|---|---|---|
| Patients aged ≥65 y | –26.4% | 3.8 | –1.7% | 1662 | 0.11 | 15298 |
| Patients who are immunocompromised | –21.2% | 4.7 | –1.4% | 2081 | 0.17 | 12597 |
| Patients with severe CDI on presentation | –19.5% | 5.1 | –1.2% | 2228 | 0.10 | 21430 |
| Patients aged ≥65 y and ≥1 previous episode in prior 6 mo | –39.7% | 2.5 | –2.6% | 587 | 0.16 | 3591 |
| Patients who are immunocompromised and ≥1 previous episode in prior 6 mo | –33.0% | 3.0 | –2.1% | 1127 | 0.23 | 4979 |
| Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo | –40.2% | 2.5 | –2.5% | 555 | 0.19 | 2938 |
| Subgroup . | Incremental Total Recurrence (Bezlotoxumab – Placebo) . | NNT to Prevent a Recurrence . | Incremental Mortality (Bezlotoxumab – Placebo) . | Incremental Costs, $ (Bezlotoxumab – Placebo) . | Incremental QALYs (Bezlotoxumab – Placebo) . | ICER, $/QALY . |
|---|---|---|---|---|---|---|
| Patients aged ≥65 y | –26.4% | 3.8 | –1.7% | 1662 | 0.11 | 15298 |
| Patients who are immunocompromised | –21.2% | 4.7 | –1.4% | 2081 | 0.17 | 12597 |
| Patients with severe CDI on presentation | –19.5% | 5.1 | –1.2% | 2228 | 0.10 | 21430 |
| Patients aged ≥65 y and ≥1 previous episode in prior 6 mo | –39.7% | 2.5 | –2.6% | 587 | 0.16 | 3591 |
| Patients who are immunocompromised and ≥1 previous episode in prior 6 mo | –33.0% | 3.0 | –2.1% | 1127 | 0.23 | 4979 |
| Patients with severe CDI on presentation and ≥1 previous episode in prior 6 mo | –40.2% | 2.5 | –2.5% | 555 | 0.19 | 2938 |
Abbreviations: CDI, Clostridium difficile infection; ICER, incremental cost-effectiveness ratio; NNT, number needed to treat; QALY, quality-adjusted life-year.
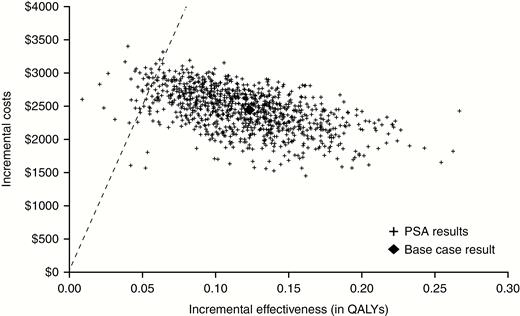
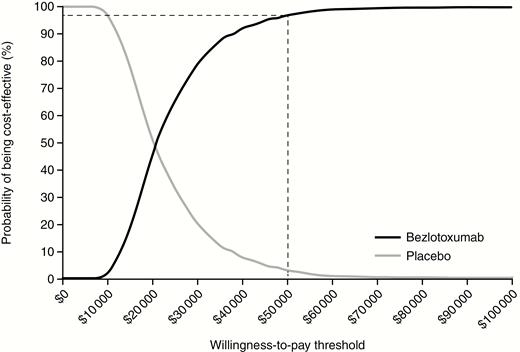
Deterministic sensitivity analyses on the entire clinical trial population are reported in Supplementary Table A7 and showed that the ICER was most sensitive to 180-day mortality rate, recurrence rates, and quality-of-life multipliers for post-CDI health state. The probabilistic sensitivity analysis conducted for the entire population and involving 1000 simulations is presented as a cost-effectiveness plane (Figure 2), which shows the point estimate and narrow distribution of the results, reflecting the confidence around the ICER. Moreover, the cost-effectiveness acceptability curve showed that the addition of bezlotoxumab to SoC has a 96.7% probability of being cost-effective at a willingness-to-pay threshold of $50 000/QALY gained (Figure 3).
Cost-effectiveness plane: entire clinical trial population (dashed line indicates willingness-to-pay threshold of $50000/quality-adjusted life-year gained). Abbreviations: PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year.
Cost-effectiveness acceptability curve: entire clinical trial population (dashed line indicates willingness-to-pay threshold of $50000/quality-adjusted life-year gained).
DISCUSSION
CDI is an important cause of both morbidity and mortality, with the initial episode often compounded by recurrence [27]. Moreover, CDI adds significant costs and burden to the US healthcare system, with the aggregate hospital cost of treatment of CDI in the United States in 2009 reported to be $8.2 billion, representing 2.3% of total US hospital costs [11]. On average, patients with CDI spend an extra 13.6 days in hospital (range, 2.2–16 days) compared with noninfected patients [28], and increased hospital length of stay is a major contributor to increased costs. Furthermore, as CDI is difficult to eradicate from a hospital, the hospitalization of a CDI patient can lead to the spread of infection to other vulnerable inpatients. Identification of a cost-effective strategy for the prevention of recurrent CDI is thus an important clinical priority.
Bezlotoxumab is indicated to reduce the rate of recurrence of CDI [29], which is different from a drug that is solely used to treat the condition (and not prevent recurrence). Our model showed that bezlotoxumab administered in addition to SoC is cost-effective compared with placebo, in a patient population reflective of the overall MODIFY trial population, at a willingness-to-pay threshold of $50000/QALY.
More importantly, bezlotoxumab administered in addition to SoC was most cost-effective in subgroups that had a higher rate of recurrence, including those aged ≥65 years or immunocompromised, and also in all the stratified risk groups that had 1 or more episodes of CDI in the previous 6 months and were aged ≥65 years, immunocompromised, or had severe CDI. Thus, bezlotoxumab has shown high economic value in subgroups with higher burden. Both deterministic and probabilistic sensitivity analysis also demonstrated that bezlotoxumab remains cost-effective under a variety of parameter assumptions.
Our model shows that the MODIFY I/II trials underestimated the number needed to treat (NNT) to prevent an episode of recurrent CDI. This is because the clinical trial NNT is estimated after the first recurrence. In reality, patients who experience a recurrence may have subsequent recurrent episodes, which are captured in our model and may need further evaluation through well-designed clinical trials or real-world cohort analysis.
Whereas including subsequent episodes impacts our NNT to avert a recurrence, it does not impact the ICER, as the attributable cost and mortality are applied at the first recurrent episode only based on Dubberke et al [12]. Attributable mortality is one of the parameters to which the ICER is sensitive. The mortality of 10.6% is attributable to recurrent CDI, and is not mortality attributable to bezlotoxumab over placebo. The incremental mortality associated with placebo was estimated by the model to be 1.1% for the base case of the entire clinical trial population.
As with any other modeling study, the limitations of this model are mainly due to the availability of data. As many of the assumptions were based on published literature, it was important to ensure that the data were representative of the model definitions. With parameters such as recurrence, mortality, and healthcare resource use all dependent upon the baseline demographics, the severity of the episode, hospitalization cost, and length of stay, careful selection of data was critical for the model [30].
Furthermore, although the model had limited data on utilities for the acute phase of the CDI illness, the duration of the acute phase is short (14 days), and deterministic sensitivity analysis showed that the ICER was not sensitive to the utility weights for the acute phases of the disease. Most of the utility benefit accrued through the life-years gained due to mortality averted because of reduced recurrent CDI. Finally, the cost of recurrence in this model is an average of all patients who have at least 1 recurrence, yet other studies have shown that the costs of a recurrence in patients with more comorbidities or risk factors will be greater [28]. While the subgroup analyses considered both efficacy and baseline rates of recurrence, they did not account for the greater costs of recurrence, which makes the cost estimates overly conservative for these subgroups.
In conclusion, a model-based cost-effectiveness analysis has shown that, through prevention of recurrent CDI, bezlotoxumab administered together with SoC antibiotics is cost-effective compared with SoC alone. Given the urgent population-level threat of CDI identified by the US Centers for Disease Control and Prevention [31] and the limited pharmacotherapy options available to prevent recurrent CDI, bezlotoxumab presents itself as a timely intervention to reduce the burden of recurrent CDI.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Medical writing assistance was provided by K. Ian Johnson, BSc, of McCann Health, Macclesfield, United Kingdom. This assistance was funded by Merck & Co, Inc, Kenilworth, New Jersey.
Financial support. This work was supported by Merck & Co, Inc, Kenilworth, New Jersey.
Potential conflicts of interest. V. S. P., N. C., M. B. D., E. E., S. M., and Y. J. are, or were at the time of study conduct, employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, and may own stock and/or stock options in the company. E. R. D. has acted as a consultant to Merck & Co, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments