-
PDF
- Split View
-
Views
-
Cite
Cite
William F. Hartsell, Charles B. Scott, Deborah Watkins Bruner, Charles W. Scarantino, Robert A. Ivker, Mack Roach, John H. Suh, William F. Demas, Benjamin Movsas, Ivy A. Petersen, Andre A. Konski, Charles S. Cleeland, Nora A. Janjan, Michelle DeSilvio, Randomized Trial of Short- Versus Long-Course Radiotherapy for Palliation of Painful Bone Metastases, JNCI: Journal of the National Cancer Institute, Volume 97, Issue 11, 1 June 2005, Pages 798–804, https://doi.org/10.1093/jnci/dji139
Close - Share Icon Share
Abstract
Background: Radiation therapy is effective in palliating pain from bone metastases. We investigated whether 8 Gy delivered in a single treatment fraction provides pain and narcotic relief that is equivalent to that of the standard treatment course of 30 Gy delivered in 10 treatment fractions over 2 weeks. Methods: A prospective, phase III randomized study of palliative radiation therapy was conducted for patients with breast or prostate cancer who had one to three sites of painful bone metastases and moderate to severe pain. Patients were randomly assigned to 8 Gy in one treatment fraction (8-Gy arm) or to 30 Gy in 10 treatment fractions (30-Gy arm). Pain relief at 3 months after randomization was evaluated with the Brief Pain Inventory. The Wilcoxon–Mann–Whitney test was used to compare response to treatment in terms of pain and narcotic relief between the two arms and for each stratification variable. All statistical comparisons were two-sided. Results: There were 455 patients in the 8-Gy arm and 443 in the 30-Gy arm; pretreatment characteristics were equally balanced between arms. Grade 2–4 acute toxicity was more frequent in the 30-Gy arm (17%) than in the 8-Gy arm (10%) (difference = 7%, 95% CI = 3% to 12%; P = .002). Late toxicity was rare (4%) in both arms. The overall response rate was 66%. Complete and partial response rates were 15% and 50%, respectively, in the 8-Gy arm compared with 18% and 48% in the 30-Gy arm ( P = .6). At 3 months, 33% of all patients no longer required narcotic medications. The incidence of subsequent pathologic fracture was 5% for the 8-Gy arm and 4% for the 30-Gy arm. The retreatment rate was statistically significantly higher in the 8-Gy arm (18%) than in the 30-Gy arm (9%) ( P <.001). Conclusions: Both regimens were equivalent in terms of pain and narcotic relief at 3 months and were well tolerated with few adverse effects. The 8-Gy arm had a higher rate of re-treatment but had less acute toxicity than the 30-Gy arm.
Radiation therapy is quite effective in providing relief from painful bone metastases; 50%–80% of patients experience improvement in their pain, and 20%–50% of the treated patients have complete pain relief ( 1 , 2 ) . Randomized trials have previously found that a shorter course of radiation therapy (one to five treatments) may give substantial pain relief, perhaps the equivalent of that observed with longer treatment courses (10–15 treatments) ( 3 – 6 ) . This result remains controversial, and a consensus meeting on the treatment of bone metastases ( 7 ) concluded that “the relationships between radiotherapy dose and response duration in terms of pain relief and bone healing are poorly defined and require further investigation.” Despite these results, longer courses of treatment to higher total doses of radiation remain the most commonly used schedules in the United States, typically with a regimen of 30 Gy given in 10 treatment fractions over 2 weeks ( 8 – 11 ) .
A shorter course of treatment has the advantages that it is logistically much easier for patients and their families to arrange for one or two sessions rather than 10 or more daily sessions and that it has less impact on the timing of other treatments (e.g., systemic therapy). These advantages are most applicable if the shorter course of treatment is as effective as the longer course of treatment. The longer course of treatment would be preferred if there were an advantage in pain relief, decreased risk of pain recurrence, or a decrease in the risk of pathologic fracture (i.e., a fracture caused by the tumor).
The Radiation Therapy Oncology Group (RTOG) has previously studied various treatment fractionation regimens for palliation of bone metastases and found that shorter treatments were as effective as longer treatments in achieving pain relief ( 4 ) . However, many aspects of the study were criticized, including the use of physician (rather than patient) assessment of pain, the inclusion of a wide range of primary sites and histologic types of cancers, and the fact that narcotic relief and the incidence of re-treatment were not taken into consideration ( 12 ) .
The RTOG 9714 trial was undertaken to evaluate the effectiveness of different radiation therapy regimens with more sensitive evaluation tools and in a more homogeneous population of patients. Measurement of the physical, psychological, and social dimensions of quality of life and pain were assessed with a general quality-of-life instrument, the Functional Assessment of Cancer Therapy ( 13 ) . A patient self-assessment instrument—the Brief Pain Inventory ( 14 ) —was used to assess severity, location, chronicity, degree of relief as a result of therapy, perceived availability of relief, and interference with daily activities. The worst pain score of this index is the most highly correlated with interference with enjoyment of daily activities, with mild pain indicated by scores of 1–4, moderate pain with scores of 5–6, and severe pain with scores of 7–10 ( 15 ) .
The primary objective of the study was to determine whether 8 Gy of radiation therapy delivered in a single treatment fraction provides pain and narcotic relief that is equivalent to 30 Gy of radiation therapy delivered in 10 treatment fractions for patients with painful bone metastases. We limited the trial to patients whose primary cancers were breast and prostate cancers to evaluate a more relatively homogeneous group of patients with sufficient life expectancy to adequately evaluate length of response and the risk of pathologic fracture. This report presents the initial response to treatment (i.e., 3 months) for this group of patients.
P ATIENTS AND M ETHODS
Patient Eligibility and Treatment
This cooperative-group, prospective, phase III, randomized study was conducted by the RTOG and the North Central Cancer Treatment Group. Eligibility requirements included age of 18 years or older, histologically proven primary malignancy of breast or prostate, radiographic evidence of bone metastasis, pain corresponding to the area of bone metastasis, a Karnofsky performance status of at least 40, and an estimated life expectancy of at least 3 months. Pain was assessed with the Worst Pain Score from the Brief Pain Inventory, requiring a score of at least 5 on a scale of 10 (or a score of less than 5 but taking narcotic medications with a daily oral morphine equivalent dose of at least 60 mg), i.e., moderate to severe pain. Eligible treatment sites were classified as weight-bearing sites (i.e., pelvis [excluding pubis], femur, tibia, sacrum, and/or sacroiliac joints) or non-weight-bearing sites. Patients with up to three separate sites of painful metastases were eligible for the study. Patients receiving bisphosphonates or systemic therapy (hormonal therapy, chemotherapy, immunotherapy, or systemic radioisotope therapy) were eligible as long as there had been no introduction of any systemic therapy within the 30 days before entry into the study. A signed study-specific informed consent was required before randomization. This study was approved by the institutional review board at each participating institution.
Patients were ineligible if the painful area had received prior radiation therapy or palliative surgery, if there was pathologic fracture or impending fracture of the treatment site, or if there was planned surgical fixation of the bone. Patients with clinical or radiographic evidence of spinal cord or cauda equina compression and/or effacement were not eligible.
Required information before randomization included history and physical examination, Karnofsky performance status, radiographically documented bone metastases within 8 weeks before randomization, and completed Brief Pain Inventory, Functional Assessment of Cancer Therapy, and Health Utilities Index III assessments.
Patients were randomly assigned to one of two treatment schedules: 3 Gy of radiation therapy delivered in 10 fractions over 2 weeks to a total of 30 Gy (30-Gy arm) or 8 Gy of radiation therapy delivered in one fraction (8-Gy arm). Treatment allocation used a randomized permuted block design, with balance maintained within each institution. Patients were stratified by number of painful sites (solitary or multiple), treatment site (weight-bearing site or non-weight-bearing site), initial worst pain score (<5 [with ≥60 mg of morphine or equivalent], 5–6, or 7–10), and use of bisphosphonates (yes or no). Simulation of treatment fields was required before treatment; the treatment volume included the radiographic abnormality with at a margin of at least 2 cm, but treatment of the entire bone was not required.
Statistical Methods
The primary null hypothesis was that, for patients with painful bone metastases, pain and narcotic relief from 8 Gy of radiation therapy in a single treatment fraction is equivalent to that from 30 Gy of radiation therapy in 10 treatment fractions. The study was designed to show equivalence if at least 36% of patients in the 8-Gy arm achieved complete pain and narcotic relief (Brief Pain Inventory worst pain score = 0 and not using any narcotic pain medications at 3 months after randomization). If we used Blackwelder's method ( 16 ) and assumed an ineligible or nonevaluable (no data submitted) rate of 10%, then a total of 938 patients would be required to detect a greater than 21.7% change in complete pain and narcotic relief with a statistical significance level of .05 and a statistical power of 90%. The Wilcoxon–Mann–Whitney test was used to test the primary null hypothesis ( 17 ) . Overall survival was estimated by the Kaplan–Meier method. The log-rank statistic was used to test for differences ( 18 , 19 ) . Re-treatment rates were estimated by the cumulative incidence method and differences between the arms were tested by using Gray's test because these methods account for competing events such as dying without being re-treated ( 20 , 21 ) . Statistical comparisons to assess the response to treatment at 3 months between the treatment arms for each stratification variable were carried out with the Wilcoxon–Mann–Whitney test. Analysis was performed by intention to treat instead of by actual treatment received.
Response was determined by follow-up questionnaires and telephone interviews with poor-compliance patients, when necessary for completeness. Questionnaires (at 2 and 4 weeks and at 2, 3, 6, 9, 12, 18, 24, 30, 36, 48, and 60 months) included the Functional Assessment of Cancer Therapy, Brief Pain Inventory, Health Utilities Index III, and the pain and narcotic scores. Time to maximal pain relief was defined as the time from the first day of irradiation until the lowest pain score for worst pain after radiation therapy. The worst pain score was used as the marker for treatment response. A complete response was defined as having no pain at 3 months after radiation therapy, a partial response was defined as a pain score that was at least two points lower than the initial response, a stable response was defined as a one-point change in pain score in either direction, and a progressive response was defined as a pain score that was at least two points higher than the initial score. All statistical tests were two-sided.
R ESULTS
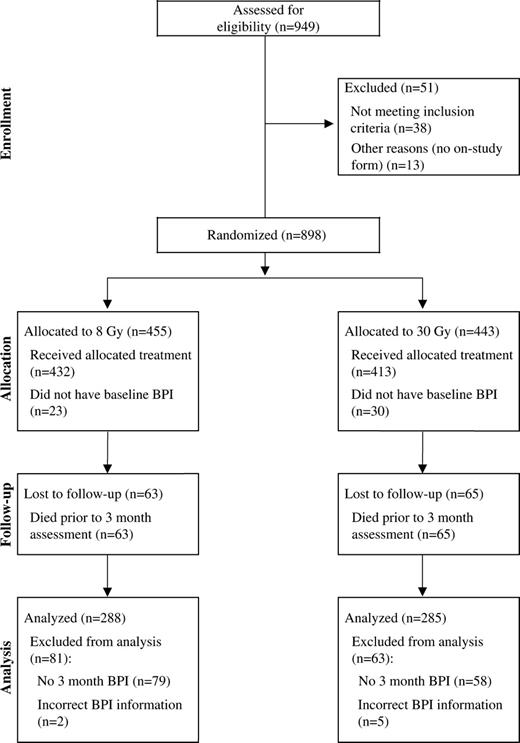
Between 1998 and 2001, a total of 949 patients were enrolled in the study, of whom 898 were considered eligible (455 in the 8-Gy arm and 443 in the 30-Gy arm) ( Fig. 1 ).Reasons for ineligibility included: no on-study form (13 patients), no pretreatment Brief Pain Inventory (nine patients), systemic therapy initiated within 30 days of study entry (nine patients), pathologic fracture in treatment site (five patients), no imaging before treatment (four patients), worst pain score of less than 5 and narcotic dose of less than 60 mg of morphine equivalent (four patients), cord compression (two patients), prior radiotherapy to treatment site (three patients), and no metastases and/or no pain at treatment site (three patients). One patient withdrew from the study before treatment. Two patients were ineligible for more than one reason.
Patient characteristics were well balanced between the two arms ( Table 1 ). Treatment was allowed for spine lesions as long as there was no clinical or radiographic evidence of spinal cord or cauda equina compression. The cervical spine was treated in 47 (5.2%) of the 898 patients, the thoracic spine was treated in 174 (19.4%) patients, and the lumbar spine was treated in 239 (26.6%) patients. There was excellent patient compliance with completion of the initial pain and quality of life questionnaires. Both the Functional Assessment of Cancer Therapy (FACT) and Health Utilities Index III were completed by 98% of patients. The average FACT score before the start of therapy was approximately 72 in both arms (possible range = 0–112, with higher score representing better quality of life), indicating balance in pretreatment distribution. For comparison, in a randomized trial evaluating epoetin in anemic patients with incurable cancer, the median baseline FACT scores were 70.6–72.1 ( 22 ) . Quality-of-life and health utilities data will be reported elsewhere ( 23 ) .
Pretreatment characteristics *
| Characteristic . | 8-Gy arm (n = 455) . | 30-Gy arm (n = 443) . |
|---|---|---|
| Age, y | ||
| Mean | 65.5 | 65.1 |
| Median | 67.5 | 67 |
| Range | 33–92 | 31–91 |
| Sex, No. of patients (%) | ||
| Male | 222 (49) | 223 (50) |
| Female | 233 (51) | 220 (50) |
| Race, No. of patients (%) | ||
| White | 343 (75) | 344 (78) |
| Hispanic | 18 (4) | 24 (5) |
| Black | 83 (18) | 67 (15) |
| Asian | 8 (2) | 5 (1) |
| Native American | 2 (<1) | 1 (<1) |
| Other | 1 (<1) | 1 (<1) |
| Prefers not to answer | 0 | 1 (<1) |
| KPS, No. of patients (%) | ||
| 40–60 | 104 (23) | 104 (23) |
| 70–80 | 255 (56) | 229 (52) |
| 90–100 | 96 (21) | 106 (24) |
| Unknown | 0 | 4 (1) |
| Painful sites, No. of patients (%) | ||
| Solitary | 271 (60) | 236 (53) |
| Multiple | 184 (40) | 207 (47) |
| Treatment site, No. of patients (%) | ||
| Weight bearing | 256 (56) | 246 (56) |
| Non–weight bearing | 199 (44) | 197 (44) |
| Worst pain score (BPI), No. of patients (%) | ||
| <5 | 12 (3) | 11 (2) |
| 5–6 | 113 (25) | 113 (26) |
| 7–10 | 330 (73) | 319 (72) |
| Receiving pamidronate/bisphosphonates, No. of patients (%) | ||
| No | 330 (73) | 325 (73) |
| Yes | 125 (27) | 118 (27) |
| Characteristic . | 8-Gy arm (n = 455) . | 30-Gy arm (n = 443) . |
|---|---|---|
| Age, y | ||
| Mean | 65.5 | 65.1 |
| Median | 67.5 | 67 |
| Range | 33–92 | 31–91 |
| Sex, No. of patients (%) | ||
| Male | 222 (49) | 223 (50) |
| Female | 233 (51) | 220 (50) |
| Race, No. of patients (%) | ||
| White | 343 (75) | 344 (78) |
| Hispanic | 18 (4) | 24 (5) |
| Black | 83 (18) | 67 (15) |
| Asian | 8 (2) | 5 (1) |
| Native American | 2 (<1) | 1 (<1) |
| Other | 1 (<1) | 1 (<1) |
| Prefers not to answer | 0 | 1 (<1) |
| KPS, No. of patients (%) | ||
| 40–60 | 104 (23) | 104 (23) |
| 70–80 | 255 (56) | 229 (52) |
| 90–100 | 96 (21) | 106 (24) |
| Unknown | 0 | 4 (1) |
| Painful sites, No. of patients (%) | ||
| Solitary | 271 (60) | 236 (53) |
| Multiple | 184 (40) | 207 (47) |
| Treatment site, No. of patients (%) | ||
| Weight bearing | 256 (56) | 246 (56) |
| Non–weight bearing | 199 (44) | 197 (44) |
| Worst pain score (BPI), No. of patients (%) | ||
| <5 | 12 (3) | 11 (2) |
| 5–6 | 113 (25) | 113 (26) |
| 7–10 | 330 (73) | 319 (72) |
| Receiving pamidronate/bisphosphonates, No. of patients (%) | ||
| No | 330 (73) | 325 (73) |
| Yes | 125 (27) | 118 (27) |
KPS = Karnofsky performance status; BPI = Brief Pain Inventory.
Pretreatment characteristics *
| Characteristic . | 8-Gy arm (n = 455) . | 30-Gy arm (n = 443) . |
|---|---|---|
| Age, y | ||
| Mean | 65.5 | 65.1 |
| Median | 67.5 | 67 |
| Range | 33–92 | 31–91 |
| Sex, No. of patients (%) | ||
| Male | 222 (49) | 223 (50) |
| Female | 233 (51) | 220 (50) |
| Race, No. of patients (%) | ||
| White | 343 (75) | 344 (78) |
| Hispanic | 18 (4) | 24 (5) |
| Black | 83 (18) | 67 (15) |
| Asian | 8 (2) | 5 (1) |
| Native American | 2 (<1) | 1 (<1) |
| Other | 1 (<1) | 1 (<1) |
| Prefers not to answer | 0 | 1 (<1) |
| KPS, No. of patients (%) | ||
| 40–60 | 104 (23) | 104 (23) |
| 70–80 | 255 (56) | 229 (52) |
| 90–100 | 96 (21) | 106 (24) |
| Unknown | 0 | 4 (1) |
| Painful sites, No. of patients (%) | ||
| Solitary | 271 (60) | 236 (53) |
| Multiple | 184 (40) | 207 (47) |
| Treatment site, No. of patients (%) | ||
| Weight bearing | 256 (56) | 246 (56) |
| Non–weight bearing | 199 (44) | 197 (44) |
| Worst pain score (BPI), No. of patients (%) | ||
| <5 | 12 (3) | 11 (2) |
| 5–6 | 113 (25) | 113 (26) |
| 7–10 | 330 (73) | 319 (72) |
| Receiving pamidronate/bisphosphonates, No. of patients (%) | ||
| No | 330 (73) | 325 (73) |
| Yes | 125 (27) | 118 (27) |
| Characteristic . | 8-Gy arm (n = 455) . | 30-Gy arm (n = 443) . |
|---|---|---|
| Age, y | ||
| Mean | 65.5 | 65.1 |
| Median | 67.5 | 67 |
| Range | 33–92 | 31–91 |
| Sex, No. of patients (%) | ||
| Male | 222 (49) | 223 (50) |
| Female | 233 (51) | 220 (50) |
| Race, No. of patients (%) | ||
| White | 343 (75) | 344 (78) |
| Hispanic | 18 (4) | 24 (5) |
| Black | 83 (18) | 67 (15) |
| Asian | 8 (2) | 5 (1) |
| Native American | 2 (<1) | 1 (<1) |
| Other | 1 (<1) | 1 (<1) |
| Prefers not to answer | 0 | 1 (<1) |
| KPS, No. of patients (%) | ||
| 40–60 | 104 (23) | 104 (23) |
| 70–80 | 255 (56) | 229 (52) |
| 90–100 | 96 (21) | 106 (24) |
| Unknown | 0 | 4 (1) |
| Painful sites, No. of patients (%) | ||
| Solitary | 271 (60) | 236 (53) |
| Multiple | 184 (40) | 207 (47) |
| Treatment site, No. of patients (%) | ||
| Weight bearing | 256 (56) | 246 (56) |
| Non–weight bearing | 199 (44) | 197 (44) |
| Worst pain score (BPI), No. of patients (%) | ||
| <5 | 12 (3) | 11 (2) |
| 5–6 | 113 (25) | 113 (26) |
| 7–10 | 330 (73) | 319 (72) |
| Receiving pamidronate/bisphosphonates, No. of patients (%) | ||
| No | 330 (73) | 325 (73) |
| Yes | 125 (27) | 118 (27) |
KPS = Karnofsky performance status; BPI = Brief Pain Inventory.
Median survival was 9.1 months in the 8-Gy arm and 9.5 months in the 30-Gy arm ( P = .820). Overall survival was 41% at 1 year and 22% at 2 years in the 8-Gy arm, respectively, and 42% and 22% in the 30-Gy arm. Treatment was well tolerated by patients in both treatment arms, but more patients had acute toxicities (grades 2–4) in the 30-Gy arm (70 events, 17%) than in the 8-Gy arm (42 events, 10%) (difference = 7%, 95% CI = 3% to 12%; P = .002). The most common toxicity, gastrointestinal toxicity, accounted for approximately half of all acute adverse events. Only two patients, both in the 30-Gy arm, had grade 4 acute toxicities (one with emesis and one with neutropenia) ( Table 2 ). Four patients, two in each arm, experienced grade 3 late toxicity. There were no grade 4 late toxicities. The incidence of grade 2 or greater late toxicity in both arms was 4% (28 of 696 patients). The median follow-up for patients with reported toxicity is 7.6 months (range = 0.2–49 months).
Toxicity of treatment *
| . | Acute toxicity, No. of patients . | . | . | . | . | . | . | . | Late toxicity, No. of patients . | . | . | . | . | . | . | . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 8-Gy arm (n = 433) . | . | . | . | 30-Gy arm (n = 414) . | . | . | . | 8-Gy arm (n = 354) . | . | . | . | 30-Gy arm (n = 342) . | . | . | . | ||||||||||||||
| Type of toxicity . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | ||||||||||||||
| Skin | 15 | 1 | 0 | 0 | 32 | 15 | 1 | 0 | 7 | 1 | 0 | 0 | 3 | 2 | 0 | 0 | ||||||||||||||
| Lung | 0 | 0 | 2 | 0 | 3 | 4 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | ||||||||||||||
| CNS | 3 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | ||||||||||||||
| GI | 29 | 21 | 3 | 0 | 47 | 27 | 6 | 0 | 5 | 1 | 0 | 0 | 4 | 2 | 0 | 0 | ||||||||||||||
| Hematologic | 10 | 7 | 2 | 0 | 11 | 10 | 5 | 1 | 5 | 3 | 1 | 0 | 5 | 3 | 0 | 0 | ||||||||||||||
| Other | 11 | 6 | 6 | 0 | 15 | 13 | 4 | 1 | 4 | 5 | 0 | 0 | 4 | 6 | 2 | 0 | ||||||||||||||
| Maximum toxicity per patient | 43 | 31 | 11 | 0 | 65 | 55 | 13 | 2 | 10 | 11 | 2 | 0 | 11 | 13 | 2 | 0 | ||||||||||||||
| . | Acute toxicity, No. of patients . | . | . | . | . | . | . | . | Late toxicity, No. of patients . | . | . | . | . | . | . | . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 8-Gy arm (n = 433) . | . | . | . | 30-Gy arm (n = 414) . | . | . | . | 8-Gy arm (n = 354) . | . | . | . | 30-Gy arm (n = 342) . | . | . | . | ||||||||||||||
| Type of toxicity . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | ||||||||||||||
| Skin | 15 | 1 | 0 | 0 | 32 | 15 | 1 | 0 | 7 | 1 | 0 | 0 | 3 | 2 | 0 | 0 | ||||||||||||||
| Lung | 0 | 0 | 2 | 0 | 3 | 4 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | ||||||||||||||
| CNS | 3 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | ||||||||||||||
| GI | 29 | 21 | 3 | 0 | 47 | 27 | 6 | 0 | 5 | 1 | 0 | 0 | 4 | 2 | 0 | 0 | ||||||||||||||
| Hematologic | 10 | 7 | 2 | 0 | 11 | 10 | 5 | 1 | 5 | 3 | 1 | 0 | 5 | 3 | 0 | 0 | ||||||||||||||
| Other | 11 | 6 | 6 | 0 | 15 | 13 | 4 | 1 | 4 | 5 | 0 | 0 | 4 | 6 | 2 | 0 | ||||||||||||||
| Maximum toxicity per patient | 43 | 31 | 11 | 0 | 65 | 55 | 13 | 2 | 10 | 11 | 2 | 0 | 11 | 13 | 2 | 0 | ||||||||||||||
G = grade; CNS = central nervous system; GI = gastrointestinal system. Numbers in the table represent the worst toxicity experienced by each patient when we used the Radiation Therapy Oncology Group (RTOG) Acute Morbidity Criteria for any reactions occurring within the first 90 days after starting radiation therapy (acute toxicity) and the RTOG Late Morbidity Criteria of any side effects occurring after 90 days (late toxicity). For example, in the group that received a single dose of 8 Gy, grade 1 late skin toxicity (slight atrophy, pigmentation change, or some hair loss) occurred in seven of 354 patients and grade 2 late toxicity (patchy atrophy, moderate telangiectasia, or total hair loss) occurred in one patient. The remaining 346 patients had no late skin toxicity. The numbers of patients in each treatment arm are fewer than in Table 1 because 51 patients (22 on 8-Gy arm and 29 on the 30-Gy arm) did not have acute toxicity information submitted. Additional patients were lost in the late toxicity time point due to death within 90 days or no follow-up information submitted.
Toxicity of treatment *
| . | Acute toxicity, No. of patients . | . | . | . | . | . | . | . | Late toxicity, No. of patients . | . | . | . | . | . | . | . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 8-Gy arm (n = 433) . | . | . | . | 30-Gy arm (n = 414) . | . | . | . | 8-Gy arm (n = 354) . | . | . | . | 30-Gy arm (n = 342) . | . | . | . | ||||||||||||||
| Type of toxicity . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | ||||||||||||||
| Skin | 15 | 1 | 0 | 0 | 32 | 15 | 1 | 0 | 7 | 1 | 0 | 0 | 3 | 2 | 0 | 0 | ||||||||||||||
| Lung | 0 | 0 | 2 | 0 | 3 | 4 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | ||||||||||||||
| CNS | 3 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | ||||||||||||||
| GI | 29 | 21 | 3 | 0 | 47 | 27 | 6 | 0 | 5 | 1 | 0 | 0 | 4 | 2 | 0 | 0 | ||||||||||||||
| Hematologic | 10 | 7 | 2 | 0 | 11 | 10 | 5 | 1 | 5 | 3 | 1 | 0 | 5 | 3 | 0 | 0 | ||||||||||||||
| Other | 11 | 6 | 6 | 0 | 15 | 13 | 4 | 1 | 4 | 5 | 0 | 0 | 4 | 6 | 2 | 0 | ||||||||||||||
| Maximum toxicity per patient | 43 | 31 | 11 | 0 | 65 | 55 | 13 | 2 | 10 | 11 | 2 | 0 | 11 | 13 | 2 | 0 | ||||||||||||||
| . | Acute toxicity, No. of patients . | . | . | . | . | . | . | . | Late toxicity, No. of patients . | . | . | . | . | . | . | . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 8-Gy arm (n = 433) . | . | . | . | 30-Gy arm (n = 414) . | . | . | . | 8-Gy arm (n = 354) . | . | . | . | 30-Gy arm (n = 342) . | . | . | . | ||||||||||||||
| Type of toxicity . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | G1 . | G2 . | G3 . | G4 . | ||||||||||||||
| Skin | 15 | 1 | 0 | 0 | 32 | 15 | 1 | 0 | 7 | 1 | 0 | 0 | 3 | 2 | 0 | 0 | ||||||||||||||
| Lung | 0 | 0 | 2 | 0 | 3 | 4 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | ||||||||||||||
| CNS | 3 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | ||||||||||||||
| GI | 29 | 21 | 3 | 0 | 47 | 27 | 6 | 0 | 5 | 1 | 0 | 0 | 4 | 2 | 0 | 0 | ||||||||||||||
| Hematologic | 10 | 7 | 2 | 0 | 11 | 10 | 5 | 1 | 5 | 3 | 1 | 0 | 5 | 3 | 0 | 0 | ||||||||||||||
| Other | 11 | 6 | 6 | 0 | 15 | 13 | 4 | 1 | 4 | 5 | 0 | 0 | 4 | 6 | 2 | 0 | ||||||||||||||
| Maximum toxicity per patient | 43 | 31 | 11 | 0 | 65 | 55 | 13 | 2 | 10 | 11 | 2 | 0 | 11 | 13 | 2 | 0 | ||||||||||||||
G = grade; CNS = central nervous system; GI = gastrointestinal system. Numbers in the table represent the worst toxicity experienced by each patient when we used the Radiation Therapy Oncology Group (RTOG) Acute Morbidity Criteria for any reactions occurring within the first 90 days after starting radiation therapy (acute toxicity) and the RTOG Late Morbidity Criteria of any side effects occurring after 90 days (late toxicity). For example, in the group that received a single dose of 8 Gy, grade 1 late skin toxicity (slight atrophy, pigmentation change, or some hair loss) occurred in seven of 354 patients and grade 2 late toxicity (patchy atrophy, moderate telangiectasia, or total hair loss) occurred in one patient. The remaining 346 patients had no late skin toxicity. The numbers of patients in each treatment arm are fewer than in Table 1 because 51 patients (22 on 8-Gy arm and 29 on the 30-Gy arm) did not have acute toxicity information submitted. Additional patients were lost in the late toxicity time point due to death within 90 days or no follow-up information submitted.
The Brief Pain Inventory was complete in 845 patients at the time of study entry. The 3-month Brief Pain Inventory assessment was completed by 573 of the 845 patients; the reasons for missing Brief Pain Inventory at 3 months included patient death (128 patients), patient refusal or too ill to complete (32 patients), institutional error or late form (40 patients), patient not seen (36 patients), and other or unknown reasons (36 patients). A complete response was observed in 17% (93 patients) of the 573 patients, and partial response was observed in 49% (280 patients), for an overall response rate of 66% (375 of 573 patients); only 10% (55 patients) of the 573 patients had progression of pain ( Table 3 ). A 3-month Brief Pain Inventory assessment was available for 288 patients in the 8-Gy arm and 285 patients in the 30-Gy arm; the complete response and partial response rates for the 288 patients in the 8-Gy arm were 15% (44 patients) and 50% (143 patients), respectively, and for 285 patients in the 30-Gy arm were 18% (51 patients) and 48% (137 patients) ( P = .6). For patients treated to a solitary painful site , the complete response and partial response rates were 18% (29 patients) and 52% (85 patients) for the 165 patients in the 8-Gy arm and 21% (32 patients) and 51% (79 patients) for the 156 patients in the 30-Gy arm ( P = .17). At 3 months, 33% of patients no longer required narcotic medications ( Table 4 ). No difference in response at 3 months was observed between the two treatment arms when stratified by number of painful treatment sites, weight-bearing status, pretreatment pain score, or whether the patient was receiving bisphosphonates ( Table 5 ). In addition, there was no difference in response rate between the treatment arms at 3 months when we used the international consensus end points for complete response (pain score of zero with stable or reducing analgesic intake), with a complete response rate of 10% (25 patients) for the 256 patients in the 8-Gy arm and 12% (31 patients) for the 255 patients in the 30-Gy arm with 3-month BPI and adequate information on narcotic usage ( 24 ) .
Brief Pain Inventory (BPI) worst pain score and overall response to treatment at 3 months after treatment
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Parameter . | 8-Gy arm (n = 288) . | 30-Gy arm (n = 285) . | P* . | |
| BPI worst pain score | ||||
| 0 | 44 (15) | 51 (18) | .854 | |
| 1–4 | 99 (34) | 98 (34) | ||
| 5–6 | 56 (19) | 53 (19) | ||
| 7–10 | 89 (31) | 83 (29) | ||
| No answers/2 answers | 2 | 5 | ||
| Overall response type | ||||
| Complete | 44 (15) | 51 (18) | .6 | |
| Partial | 143 (50) | 137 (48) | ||
| Stable | 74 (26) | 69 (24) | ||
| Progressive | 27 (9) | 28 (10) | ||
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Parameter . | 8-Gy arm (n = 288) . | 30-Gy arm (n = 285) . | P* . | |
| BPI worst pain score | ||||
| 0 | 44 (15) | 51 (18) | .854 | |
| 1–4 | 99 (34) | 98 (34) | ||
| 5–6 | 56 (19) | 53 (19) | ||
| 7–10 | 89 (31) | 83 (29) | ||
| No answers/2 answers | 2 | 5 | ||
| Overall response type | ||||
| Complete | 44 (15) | 51 (18) | .6 | |
| Partial | 143 (50) | 137 (48) | ||
| Stable | 74 (26) | 69 (24) | ||
| Progressive | 27 (9) | 28 (10) | ||
The chi-square test was used for comparison of treatments. All statistical tests were two-sided. There were only 845 patients with a baseline BPI to use to compare with those with the month 3 BPI. Only 573 of those patients had a month 3 BPI, 128 patients died before submitting a month 3 BPI, seven patients completed the BPI incorrectly, and 137 patients did not submit a month 3 BPI.
Brief Pain Inventory (BPI) worst pain score and overall response to treatment at 3 months after treatment
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Parameter . | 8-Gy arm (n = 288) . | 30-Gy arm (n = 285) . | P* . | |
| BPI worst pain score | ||||
| 0 | 44 (15) | 51 (18) | .854 | |
| 1–4 | 99 (34) | 98 (34) | ||
| 5–6 | 56 (19) | 53 (19) | ||
| 7–10 | 89 (31) | 83 (29) | ||
| No answers/2 answers | 2 | 5 | ||
| Overall response type | ||||
| Complete | 44 (15) | 51 (18) | .6 | |
| Partial | 143 (50) | 137 (48) | ||
| Stable | 74 (26) | 69 (24) | ||
| Progressive | 27 (9) | 28 (10) | ||
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Parameter . | 8-Gy arm (n = 288) . | 30-Gy arm (n = 285) . | P* . | |
| BPI worst pain score | ||||
| 0 | 44 (15) | 51 (18) | .854 | |
| 1–4 | 99 (34) | 98 (34) | ||
| 5–6 | 56 (19) | 53 (19) | ||
| 7–10 | 89 (31) | 83 (29) | ||
| No answers/2 answers | 2 | 5 | ||
| Overall response type | ||||
| Complete | 44 (15) | 51 (18) | .6 | |
| Partial | 143 (50) | 137 (48) | ||
| Stable | 74 (26) | 69 (24) | ||
| Progressive | 27 (9) | 28 (10) | ||
The chi-square test was used for comparison of treatments. All statistical tests were two-sided. There were only 845 patients with a baseline BPI to use to compare with those with the month 3 BPI. Only 573 of those patients had a month 3 BPI, 128 patients died before submitting a month 3 BPI, seven patients completed the BPI incorrectly, and 137 patients did not submit a month 3 BPI.
Overall rates of analgesic and narcotic use at 3 months
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Drug . | 8-Gy arm (n = 318) . | 30-Gy arm (n = 310) . | P* . | |
| None | 65 (20) | 69 (22) | .483 | |
| Nonnarcotic analgesic | 40 (13) | 30 (10) | ||
| Narcotic | 213 (67) | 211 (68) | ||
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Drug . | 8-Gy arm (n = 318) . | 30-Gy arm (n = 310) . | P* . | |
| None | 65 (20) | 69 (22) | .483 | |
| Nonnarcotic analgesic | 40 (13) | 30 (10) | ||
| Narcotic | 213 (67) | 211 (68) | ||
The chi-square test was used for comparison of treatments. All statistical tests were two-sided. Seventy patients died before the month 3 time point. An additional 82 patients did not have any information at 3 months, and 126 patients did not respond to the narcotic question on the 3-month follow-up form.
Overall rates of analgesic and narcotic use at 3 months
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Drug . | 8-Gy arm (n = 318) . | 30-Gy arm (n = 310) . | P* . | |
| None | 65 (20) | 69 (22) | .483 | |
| Nonnarcotic analgesic | 40 (13) | 30 (10) | ||
| Narcotic | 213 (67) | 211 (68) | ||
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Drug . | 8-Gy arm (n = 318) . | 30-Gy arm (n = 310) . | P* . | |
| None | 65 (20) | 69 (22) | .483 | |
| Nonnarcotic analgesic | 40 (13) | 30 (10) | ||
| Narcotic | 213 (67) | 211 (68) | ||
The chi-square test was used for comparison of treatments. All statistical tests were two-sided. Seventy patients died before the month 3 time point. An additional 82 patients did not have any information at 3 months, and 126 patients did not respond to the narcotic question on the 3-month follow-up form.
Response to treatment at 3 months, as measured by the Brief Pain Inventory worst pain score, showing the response by treatment arm for each stratification variable
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Response by stratification variable . | 8-Gy arm (n = 288) . | 30-Gy arm (n = 285) . | P* . | |
| No. of painful sites | .550 | |||
| Solitary | ||||
| Complete | 29 (18) | 32 (21) | ||
| Partial | 85 (52) | 79 (51) | ||
| Stable | 40 (24) | 33 (21) | ||
| Progressive | 11 (7) | 12 (8) | ||
| Multiple | ||||
| Complete | 15 (12) | 19 (15) | ||
| Partial | 58 (47) | 58 (45) | ||
| Stable | 34 (28) | 36 (28) | ||
| Progressive | 16 (13) | 16 (12) | ||
| Treatment site | .547 | |||
| Weight bearing | ||||
| Complete | 22 (14) | 34 (22) | ||
| Partial | 80 (50) | 74 (47) | ||
| Stable | 44 (27) | 36 (23) | ||
| Progressive | 15 (9) | 14 (9) | ||
| Non–weight bearing | ||||
| Complete | 22 (17) | 17 (13) | ||
| Partial | 62 (49) | 63 (50) | ||
| Stable | 30 (24) | 33 (26) | ||
| Progressive | 13 (10) | 14 (11) | ||
| Pretreatment Worst Pain Score | .603 | |||
| 5–6 | ||||
| Complete | 17 (20) | 13 (18) | ||
| Partial | 28 (34) | 28 (38) | ||
| Stable | 25 (30) | 20 (27) | ||
| Progressive | 13 (16) | 12 (16) | ||
| 7–10 | ||||
| Complete | 23 (12) | 36 (18) | ||
| Partial | 113 (57) | 109 (53) | ||
| Stable | 48 (24) | 49 (24) | ||
| Progressive | 13 (7) | 10 (5) | ||
| <5 with ≥60 mg/day morphine | ||||
| Complete | 4 (50) | 2 (25) | ||
| Partial | 1 (13) | 0 | ||
| Stable | 1 (13) | 0 | ||
| Progressive | 2 (25) | 6 (75) | ||
| Bisphosphonate use | .547 | |||
| No | ||||
| Complete | 32 (16) | 41 (19) | ||
| Partial | 97 (48) | 97 (46) | ||
| Stable | 53 (26) | 51 (24) | ||
| Progressive | 21 (10) | 22 (10) | ||
| Yes | ||||
| Complete | 12 (14) | 10 (14) | ||
| Partial | 45 (53) | 40 (54) | ||
| Stable | 21 (25) | 18 (24) | ||
| Progressive | 7 (8) | 6 (8) | ||
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Response by stratification variable . | 8-Gy arm (n = 288) . | 30-Gy arm (n = 285) . | P* . | |
| No. of painful sites | .550 | |||
| Solitary | ||||
| Complete | 29 (18) | 32 (21) | ||
| Partial | 85 (52) | 79 (51) | ||
| Stable | 40 (24) | 33 (21) | ||
| Progressive | 11 (7) | 12 (8) | ||
| Multiple | ||||
| Complete | 15 (12) | 19 (15) | ||
| Partial | 58 (47) | 58 (45) | ||
| Stable | 34 (28) | 36 (28) | ||
| Progressive | 16 (13) | 16 (12) | ||
| Treatment site | .547 | |||
| Weight bearing | ||||
| Complete | 22 (14) | 34 (22) | ||
| Partial | 80 (50) | 74 (47) | ||
| Stable | 44 (27) | 36 (23) | ||
| Progressive | 15 (9) | 14 (9) | ||
| Non–weight bearing | ||||
| Complete | 22 (17) | 17 (13) | ||
| Partial | 62 (49) | 63 (50) | ||
| Stable | 30 (24) | 33 (26) | ||
| Progressive | 13 (10) | 14 (11) | ||
| Pretreatment Worst Pain Score | .603 | |||
| 5–6 | ||||
| Complete | 17 (20) | 13 (18) | ||
| Partial | 28 (34) | 28 (38) | ||
| Stable | 25 (30) | 20 (27) | ||
| Progressive | 13 (16) | 12 (16) | ||
| 7–10 | ||||
| Complete | 23 (12) | 36 (18) | ||
| Partial | 113 (57) | 109 (53) | ||
| Stable | 48 (24) | 49 (24) | ||
| Progressive | 13 (7) | 10 (5) | ||
| <5 with ≥60 mg/day morphine | ||||
| Complete | 4 (50) | 2 (25) | ||
| Partial | 1 (13) | 0 | ||
| Stable | 1 (13) | 0 | ||
| Progressive | 2 (25) | 6 (75) | ||
| Bisphosphonate use | .547 | |||
| No | ||||
| Complete | 32 (16) | 41 (19) | ||
| Partial | 97 (48) | 97 (46) | ||
| Stable | 53 (26) | 51 (24) | ||
| Progressive | 21 (10) | 22 (10) | ||
| Yes | ||||
| Complete | 12 (14) | 10 (14) | ||
| Partial | 45 (53) | 40 (54) | ||
| Stable | 21 (25) | 18 (24) | ||
| Progressive | 7 (8) | 6 (8) | ||
The Wilcoxon–Mann–Whitney test was used for comparison of treatment groups. All statistical tests were two-sided.
Response to treatment at 3 months, as measured by the Brief Pain Inventory worst pain score, showing the response by treatment arm for each stratification variable
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Response by stratification variable . | 8-Gy arm (n = 288) . | 30-Gy arm (n = 285) . | P* . | |
| No. of painful sites | .550 | |||
| Solitary | ||||
| Complete | 29 (18) | 32 (21) | ||
| Partial | 85 (52) | 79 (51) | ||
| Stable | 40 (24) | 33 (21) | ||
| Progressive | 11 (7) | 12 (8) | ||
| Multiple | ||||
| Complete | 15 (12) | 19 (15) | ||
| Partial | 58 (47) | 58 (45) | ||
| Stable | 34 (28) | 36 (28) | ||
| Progressive | 16 (13) | 16 (12) | ||
| Treatment site | .547 | |||
| Weight bearing | ||||
| Complete | 22 (14) | 34 (22) | ||
| Partial | 80 (50) | 74 (47) | ||
| Stable | 44 (27) | 36 (23) | ||
| Progressive | 15 (9) | 14 (9) | ||
| Non–weight bearing | ||||
| Complete | 22 (17) | 17 (13) | ||
| Partial | 62 (49) | 63 (50) | ||
| Stable | 30 (24) | 33 (26) | ||
| Progressive | 13 (10) | 14 (11) | ||
| Pretreatment Worst Pain Score | .603 | |||
| 5–6 | ||||
| Complete | 17 (20) | 13 (18) | ||
| Partial | 28 (34) | 28 (38) | ||
| Stable | 25 (30) | 20 (27) | ||
| Progressive | 13 (16) | 12 (16) | ||
| 7–10 | ||||
| Complete | 23 (12) | 36 (18) | ||
| Partial | 113 (57) | 109 (53) | ||
| Stable | 48 (24) | 49 (24) | ||
| Progressive | 13 (7) | 10 (5) | ||
| <5 with ≥60 mg/day morphine | ||||
| Complete | 4 (50) | 2 (25) | ||
| Partial | 1 (13) | 0 | ||
| Stable | 1 (13) | 0 | ||
| Progressive | 2 (25) | 6 (75) | ||
| Bisphosphonate use | .547 | |||
| No | ||||
| Complete | 32 (16) | 41 (19) | ||
| Partial | 97 (48) | 97 (46) | ||
| Stable | 53 (26) | 51 (24) | ||
| Progressive | 21 (10) | 22 (10) | ||
| Yes | ||||
| Complete | 12 (14) | 10 (14) | ||
| Partial | 45 (53) | 40 (54) | ||
| Stable | 21 (25) | 18 (24) | ||
| Progressive | 7 (8) | 6 (8) | ||
| . | No. of patients (%) . | . | . | |
|---|---|---|---|---|
| Response by stratification variable . | 8-Gy arm (n = 288) . | 30-Gy arm (n = 285) . | P* . | |
| No. of painful sites | .550 | |||
| Solitary | ||||
| Complete | 29 (18) | 32 (21) | ||
| Partial | 85 (52) | 79 (51) | ||
| Stable | 40 (24) | 33 (21) | ||
| Progressive | 11 (7) | 12 (8) | ||
| Multiple | ||||
| Complete | 15 (12) | 19 (15) | ||
| Partial | 58 (47) | 58 (45) | ||
| Stable | 34 (28) | 36 (28) | ||
| Progressive | 16 (13) | 16 (12) | ||
| Treatment site | .547 | |||
| Weight bearing | ||||
| Complete | 22 (14) | 34 (22) | ||
| Partial | 80 (50) | 74 (47) | ||
| Stable | 44 (27) | 36 (23) | ||
| Progressive | 15 (9) | 14 (9) | ||
| Non–weight bearing | ||||
| Complete | 22 (17) | 17 (13) | ||
| Partial | 62 (49) | 63 (50) | ||
| Stable | 30 (24) | 33 (26) | ||
| Progressive | 13 (10) | 14 (11) | ||
| Pretreatment Worst Pain Score | .603 | |||
| 5–6 | ||||
| Complete | 17 (20) | 13 (18) | ||
| Partial | 28 (34) | 28 (38) | ||
| Stable | 25 (30) | 20 (27) | ||
| Progressive | 13 (16) | 12 (16) | ||
| 7–10 | ||||
| Complete | 23 (12) | 36 (18) | ||
| Partial | 113 (57) | 109 (53) | ||
| Stable | 48 (24) | 49 (24) | ||
| Progressive | 13 (7) | 10 (5) | ||
| <5 with ≥60 mg/day morphine | ||||
| Complete | 4 (50) | 2 (25) | ||
| Partial | 1 (13) | 0 | ||
| Stable | 1 (13) | 0 | ||
| Progressive | 2 (25) | 6 (75) | ||
| Bisphosphonate use | .547 | |||
| No | ||||
| Complete | 32 (16) | 41 (19) | ||
| Partial | 97 (48) | 97 (46) | ||
| Stable | 53 (26) | 51 (24) | ||
| Progressive | 21 (10) | 22 (10) | ||
| Yes | ||||
| Complete | 12 (14) | 10 (14) | ||
| Partial | 45 (53) | 40 (54) | ||
| Stable | 21 (25) | 18 (24) | ||
| Progressive | 7 (8) | 6 (8) | ||
The Wilcoxon–Mann–Whitney test was used for comparison of treatment groups. All statistical tests were two-sided.
We investigated the incidence of pathologic fractures because such fractures tend to lower the quality of life of these patients. The incidence of pathologic fracture within the treatment field (or within plus adjacent to treatment field) was 5% and 4% for patients in the 8-Gy arm and in the 30-Gy arm, respectively. An additional 3%–4% of patients had fractures adjacent to the treatment site.
The decision to re-treat a patient was left to the discretion of the treating physician. A statistically significant difference was observed in retreatment rates between the two arms, with twice as many patients in the 8-Gy arm receiving retreatment (3-year retreatment rates: 18% [76 of the 449 patients] in the 8-Gy arm and 9% [33 of the 432 patients] in the 30-Gy arm; P <.001). The difference in retreatment rates was apparent by 3 months after the initial treatment. Most of the retreatment was given in the first 9 months after the initial treatment, and retreatment was rarely performed more than 1 year after the initial treatment.
A treatment compliance review was performed for a random sample of 30% of the eligible patients. Of these, 87.1% were within protocol guidelines or with minor deviations, 7.0% had incomplete treatment or major deviations, and 5.9% were not evaluable for compliance because of incomplete data.
D ISCUSSION
We found that external beam radiation therapy was effective at palliating pain from bone metastases, with complete or partial improvement in pain observed at 3 months after randomization in 66% (375 patients) of the 573 patients. At 3 months of follow-up, we found no difference between the response of patients in the arm receiving 30 Gy in 10 treatment fractions and in the arm receiving 8 Gy in a single treatment fraction, in terms of pain relief, narcotic relief, or pathologic fracture incidence, regardless of stratification used in the analysis. Treatment was well tolerated with few adverse effects.
The RTOG 74-02 trial compared differing dose levels of radiation therapy for palliation of patients with bone metastases. The doses ranged from 1500 cGy in five treatment fractions to 3000 cGy in 10 treatment fractions for multiple metastases and from 2000 cGy in five treatment fractions to 4050 cGy in 15 treatment fractions for patients with a solitary metastasis ( 4 ) . Pain was assessed by the treating physician using descriptors of no pain or mild, moderate, or severe pain. Overall, 89% eventually experienced at least minimal relief of pain, with 53% obtaining complete relief and another 30% experiencing partial relief. There was no statistically significant difference in pain relief rates among the differing treatment schedules. Patients with the most severe pain before treatment (highest pain scores) were statistically significantly less likely to have minimal or complete relief of pain than were patients with lower initial pain scores. The study was later reanalyzed by including improvement in narcotic score, improvement in combined pain and narcotic scores, and incidence of retreatment of the same site ( 12 ) . In the reanalysis, the number of fractions of radiation used was statistically significantly correlated with retreatment given, complete pain relief before retreatment, and complete relief by combined pain and narcotic score, suggesting that the protracted course of treatment was associated with improved outcome.
There have been multiple randomized comparisons of one or a few treatments to more standard, longer courses of radiation therapy for palliation of bone metastases ( 3 , 5 , 25 – 29 ) . Most of these studies have shown no statistically significant difference in pain relief between shorter-duration, lower-dose treatments and longer-duration, higher-dose treatments. Ratanatharathorn et al. ( 30 ) reviewed many of these studies and concluded that higher-dose, longer-course regimens provided better pain outcomes than low-dose regimens. In contrast, Wu et al. ( 31 ) performed a meta-analysis of studies comparing single versus multiple fractions of radiotherapy for palliation of painful bone metastases. They found a complete response rate of 32%–33%, an overall response rate of 72%–73%, and no difference in response rates comparing a single treatment with multiple treatments. The primary difference between the two arms was the higher rate of retreatment in the patients receiving a single fraction (11%–25%) compared with those receiving multiple fractions (0%–12%). In a meta-analysis of randomized trials comparing single-fraction radiation therapy regimens with multifraction regimens for palliation of metastatic bone pain, Sze et al. ( 32 ) found that both regimens resulted in equivalent levels of pain relief but in different rates of re-treatment and pathologic fractures between arms.
Why should a lower dose of radiotherapy be as effective as higher doses in palliating bone pain? If the response depends solely on decreasing the tumor cell burden, then the higher-dose regimens should be more effective than the lower-dose regimens. However, if the response to radiation depends (at least in part) on effects in normal tissues, the total dose may not be as important. Results of two studies ( 33 , 34 ) suggest that osteolysis and bone resorption are mediated through the RANK (i.e., receptor activator of nuclear factor κB) signaling pathway. The target for inhibition of bone resorption may be bone growth factors or cytokines derived from bone matrix or osteoclasts, and inhibition of tumor-associated osteolysis may reduce the tumor cell burden within bone ( 34 ) . The effectiveness of radiation therapy may be associated more with its impact on osteoclasts and the RANK signaling pathway than with the number of cells that are directly killed. This hypothesis is supported by the results of Hoskin et al. ( 35 ) that showed that the level of pain relief after radiotherapy for painful bone metastases was associated with persistently low urinary concentrations of pyridinoline and deoxypyridinoline (markers of bone resorption). Thus, response to treatment appears to depend on multiple factors, not just a reduction in tumor burden by direct killing of the cells.
The complete response rate in the RTOG 9714 trial was 16%, substantially lower than the previous RTOG study. The reasons for this difference may include the assessment method used and the severity of pain or extent of disease. For the RTOG 7402 trial, physicians scored pain with a four-point scale, whereas patients in our study scored pain by use of a more sensitive 10-point scale in the Brief Pain Inventory. In addition, the cohort of patients treated in our study is different from that treated 25 or more years ago. Although there were few systemic therapy options during the RTOG 7402 trial, second-, third-, and fourth-line chemotherapy options are currently available for breast cancer. In addition, multiple hormonal manipulations are available for the treatment of both breast and prostate cancer, and bisphosphonates are used in many of these patients. Pain control is better understood, with much more emphasis on adequate pain management now than 25 years ago. Thus, the patients who are referred for palliative radiation therapy now may have more widespread disease that has become resistant to other therapies, as reflected in our study by the severity of pain scores (72% of the patients in our study had severe pain at study entry) and the Karnofsky performance status scores (nearly 25% of the patients in our study had Karnofsky performance status scores of 60 or lower, and more than 75% had Karnofsky performance status scores of 80 or lower). The quality-of-life assessments also show that the patients in our study have many symptoms. Although this group of patients was ill with moderate-to-severe pain before treatment with radiation therapy, a substantial proportion had improvement in pain 3 months after treatment, and nearly one-third no longer required narcotic pain medication. The results may have been better if patients were treated earlier in the course of their disease, since previous studies have shown that patients with moderate pain are more likely to respond to treatment than those with severe pain ( 4 ) .
The only difference in outcomes between the two arms was the rate of re-treatment, with substantially more patients in the 8-Gy arm receiving retreatment than in the 30-Gy arm. This observation may be an indication that the 8-Gy treatment is less effective than the longer course of 30 Gy in 10 treatment fractions. However, rates of pain relief, narcotic use, and pathologic fracture incidence were equivalent in the two treatment arms. There may be other factors involved in the decision to re-treat a patient, such as potential physician bias ( 36 ) . There may be more willingness to give another treatment after a single-dose treatment than after a higher-dose treatment, especially retreating areas adjacent to sensitive critical normal structures (such as spinal cord, bowels, or lungs). There may be less willingness to give another treatment after a treatment of 30 Gy in 10 fractions because of the higher acute toxicity associated with that regimen. Even if there is a real increase in the need for retreatment among patients receiving a single-dose treatment, this problem may be counterbalanced by the reduced rate of acute toxicity in these patients.
Our study has several limitations. We included only patients with metastases from primary breast or prostate cancers to allow an adequate follow-up period to assess response and toxicity, because these patients tend to survive longer than patients with bone metastases from other primary sites. However, the outcomes may be different for patients with bone metastases from another primary site. A second limitation of the study involves completion of the assessment tool. The Brief Pain Inventory was completed by only 573 (67.8%) of the 845 patients at the 3-month assessment point. As would be expected in this group of patients, 160 of the 845 patients had died or were too ill to complete the form at 3 months. Thus, the Brief Pain Inventory was completed by 573 (83.6%) of the 685 patients who were alive and able to complete the form.
A consensus statement from the Second Workshop on Palliative Radiotherapy and Symptom Control in 2000 ( 37 ) confirmed the efficacy of radiation therapy, even with a single treatment, in palliating painful bone metastases. There is increasing evidence that a single 8-Gy dose provides pain relief equivalent to longer courses of palliative treatment, although the short course of treatment is associated with a higher rate of retreatment. Further analysis of data from the RTOG 9714 trial should yield important information on quality of life, health utilities (i.e., patient preferences for specific health states or treatments), and economic end points. These data will help determine whether a single dose of 8 Gy should become the standard treatment for palliation of localized painful bone metastases.
Supported by grant numbers RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 from the National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
References
Bates T. A review of local radiotherapy in the treatment of bone metastases and cord compression.
Maher EJ. The use of palliative radiotherapy in the management of breast cancer.
Price P, Hoskin PJ, Easton D, Austin D, Palmer SG, Yarnold JR. Prospective randomized trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases.
Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases. Final results of the study by the Radiation Therapy Oncology Group.
Madsen EL. Painful bone metastasis: Efficacy of radiotherapy assessed by the patients: a randomized trial comparing 4 Gy × 6 versus 10 Gy × 2.
Crellin AM, Marks A, Maher EJ. Why don't British radiotherapists give single fractions of radiotherapy for bone metastases?
Bates T, Yarnold JR, Blitzer P, Nelson OS, Rubin P, Maher EJ. Bone metastasis consensus statement.
Coia LR, Hanks GE, Martz K, Steinfeld A, Diamond JJ, Kramer S. Practice patterns of palliative care for the United States 1984–1985.
Maher EJ, Coia L, Duncan G, Lawton PA. Treatment strategies in advanced and metastatic cancer: differences in attitude between the USA, Canada and Europe.
Hartsell WF, Shah AB, Graney M, Kun LE. Palliation of bone metastases in the USA: A survey of patterns of practice. Support Care Cancer
Ben-Josef E, Shamsa F, Williams AO, Porter AT. Radiotherapeutic management of osseous metastases: a survey of current patterns of care.
Blitzer P. Reanalysis of the RTOG study of the palliation of symptomatic osseous metastases.
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy Scale: Development and validation of the general measure.
Cleeland CS, Ryan M. Pain assessment: global use of the Brief Pain Inventory.
Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function.
Blackwelder WC. Proving the null hypothesis in clinical trials.
Conover WJ. (
Kaplan EL, Meier P. Nonparameteric estimation from incomplete observations.
Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration.
Kalbfleish JD, Prentice RL. The statistical analysis of failure time data. New York (NY): John Wiley & Sons;
Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk.
Witzig TE, Silberstein PT, Loprinzi CL, Sloan JA, Novotny PJ, Mailliard JA, et al: Phase III, Randomized, Double-Blind Study of Epoetin Alfa Versus Placebo in Anemic Patients With Cancer Undergoing Chemotherapy. J Clin Oncol
Bruner DW, Winter K, Hartsell W, Konski A, Curran W, Roach III, M: Prospective health-related quality of life valuations (utilities) of 8 Gy in 1 fraction vs 30 Gy in 10 fractions for palliation of painful bone metastases: Preliminary results of RTOG 97–14.
Chow E, Wu JS, Hoskin P, Coia LR, Bentzen SM, Blitzer PH. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases.
Cole DJ. A randomized trial of a single treatment versus conventional fractionation in the palliative radiotherapy of painful bone metastases.
Nielsen OS, Bentzen SM, Sandberg E, Gadeberg CC, Timothy AR. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases.
Niewald M, Tkocz HJ, Abel U, Scheib T, Walter K, Nieder C, et al. Rapid course radiation therapy versus more standard treatment. A randomized trial for bone metastases.
Okawa T, Kita M, Goto M, Nishijima H, Miyaji N. Randomized prospective clinical trial of small, large and twice-a-day fraction radiotherapy for painful bone metastases.
Rasmusson B, Velborg I, Jenson AB, Andersson M, Banning AM, Hoffmann T, et al. Irradiation of bone metastases in breast cancer patients: A randomized study with 1 year follow-up.
Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastases: review and critical analysis of random allocation trials of local field treatment.
Wu JS, Wong R, Johnston M, Bezjak A, Whelan T. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases.
Sze WM, Shelley MD, Held I, Wilt TJ, Mason MD. Palliation of Metastatic Bone Pain: Single Fraction versus Multifraction Radiotherapy—A Systematic Review of Randomised Trials.
Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ. Osteoprotegrin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation.
Hoskin PJ, Stratford MR, Folkes LK, Regan J, Yarnold JR. Effect of local radiotherapy for bone pain on urinary markers of osteoclast activity.
Chow E, Lutz S, Beyene J. A single fraction for all, or an argument for fractionation tailored to fit the needs of each individual patient with bone metastases.