-
PDF
- Split View
-
Views
-
Cite
Cite
Takashi Sano, Yoshiyuki Hiki, Tohru Kokubo, Hitoo Iwase, Hidekazu Shigematsu, Yutaka Kobayashi, Enzymatically deglycosylated human IgA1 molecules accumulate and induce inflammatory cell reaction in rat glomeruli, Nephrology Dialysis Transplantation, Volume 17, Issue 1, January 2002, Pages 50–56, https://doi.org/10.1093/ndt/17.1.50
Close - Share Icon Share
Abstract
Background. Previously, we have been able to isolate IgA1 from IgA nephropathy (IgAN) patients, that could accumulate in rat glomeruli (glomerulophilic IgA1). The ‘glomerulophilic IgA1’ was determined to be under‐O‐glycosylated in its hinge region, suggesting that under‐O‐glycosylation in the IgA1 hinge region plays a role in its glomerular deposition in IgAN. To confirm this, the accumulation of enzymatically under‐glycosylated IgA1 in rat kidney was examined.
Methods. Human IgA1 was isolated from healthy individuals by Jacalin‐affinity chromatography. Desialylated (deS IgA1) or further degalactosylated IgA1 (deS/deGal IgA1) molecules were then prepared using neuraminidase and β‐galactosidase. Two or five mg of IgA1 were injected into the left renal artery of Wistar rats. The rats were sacrificed at various time intervals (3, 9, 24 h) and the perfused part of the renal cortex was removed for immunofluorescence and for light and electron microscopy.
Results. Distinct amounts of deS IgA1 and deS/deGal IgA1 were observed in rat glomeruli. On the other hand, untreated IgA1 molecules (native IgA1) did not show any obvious accumulation. In rats injected with under‐glycosylated IgA1, accumulation of polymorphonuclear cells (PMN) was also observed.
Conclusions. These results confirmed that under‐glycosylation of IgA1 played an important role in the glomerular accumulation of IgA1, which was followed by infiltration of PMN into glomeruli.
Introduction
IgA nephropathy is a common disease characterized by predominant deposits of IgA1 in the renal mesangium [1]. Although IgA deposition in glomeruli has been ascribed to circulating immune complexes containing IgA antibodies [2], its actual mechanism has not yet been fully understood.
The human IgA1 molecule is known to have a unique glycosylated hinge that possesses O‐glycan side chains [3]. We have reported on the role of the asialo‐galactosylβ1,3N‐acethyl‐galactosamine (asialo‐Galβ1,3GalNAc) residue in the formation of macromolecular IgA1 as a result of the conformational instability of the IgA1 molecule [4,5]. The IgA1–IgA1 interaction found in IgAN [6] was inhibited by the synthesized IgA1 hinge‐peptide core as well as by Galβ1,3GalNAc [7]. These observations pointed to the possibility that glomerular IgA deposition might occur not only due to IgA immune complexes but also due to the non‐immunological formation of macromolecular IgA1 induced by the abnormal O‐glycosylation in the IgA1 hinge.
We reported earlier that enzymatically under‐glycosylated IgA1 resulted in non‐covalent self‐aggregation and a significant increase in in vitro adhesion to extracellular matrix (ECM) proteins such as fibronectin, laminin and type IV collagen [8]. Most recently, we have been able to isolate IgA1 that could accumulate in rat glomeruli from IgAN patients. This ‘glomerulophilic IgA1’ was found to be under‐O‐glycosylated in its hinge region [9].
Therefore, we investigated the involvement of the under‐glycosylation of the IgA1 molecule in the non‐immunological glomerular accumulation of IgA1 in vivo. The aim of this study was to confirm the accumulation of the enzymatically under‐glycosylated IgA1 from healthy human individuals in rat kidney.
Materials and methods
Preparation of serum IgA1
Serum IgA1 was purified from healthy human subjects by Jacalin‐affinity chromatography [10]. Approximately 40 ml of serum was applied to a Jacalin agarose column (20 ml; Vector Laboratories, Burlingame, CA, USA). After washing with 0.01 M phosphate buffer, pH 7.5, 0.15 M NaCl (PBS) containing 0.8 M glucose to remove non‐specific binding, Jacalin‐binding protein was eluted with 0.1 M melibiose in PBS. The solution containing IgA1 was then dialysed against PBS.
Deglycosylation of normal serum IgA1
Purified IgA1 was enzymatically digested with neuraminidase (NA) from Arthrobacter ureafaciens (1.0 U/100 μl; Boehringer Mannheim GmbH) and β‐galactosidase (GA) from bovine testis (1.0 U/ml; Sigma Chemical Co., St Louis, MO, USA) according to the following procedure based on our previous study (8). The purified IgA1 from healthy individuals was lyophilized and then divided into three samples of 15 mg each. The first sample, to be used as control, was dissolved in 3 ml of 0.2 M sodium acetate buffer, pH 5.0 (SAB), without adding any enzymes. The second sample was dissolved in SAB, and 5 μl of NA was added to remove NeuAc from the IgA1. The third sample was dissolved in SAB, and 5 μl of NA and 50 μl of GA were added to release NeuAc and Gal. The enzyme‐containing IgA1 samples were incubated overnight at 37°C and then dialysed against distilled water and lyophilized. The non‐treated, NA‐treated and NA+GA‐treated IgA1 molecules were named ‘native’, ‘deS’ and ‘deS/deGal IgAs’, respectively.
Enzyme‐linked immunosorbent assay (ELISA) using peanut agglutinin and Vicia villosa lectins
To determine whether deglycosylation actually occurred, binding assays (ELISA) of the deglycosylated IgA1 to peanut agglutinin (PNA) and Vicia villosa (VV) lectin were performed. PNA and VV lectins are well known to specifically recognize asialo‐Galβ1,3GalNAc residues and terminal GalNAc residues, respectively [11,12]. Microtitration plates (Linbro/Titertec, A Flow General Company, McLean, IL, USA) were coated with native, deS, and deS/deGal IgA1 diluted to a concentration of 5 μg/ml. The untreated sites were blocked with PBS containing 1% bovine serum albumin (BSA, Sigma). 100 μl of peroxidase‐labelled PNA lectin (2 μg/ml, Seikagaku Co. Tokyo, Japan) and peroxidase‐labelled VV lectin (2 μg/ml, Sigma) were placed in the IgA1 coated and non‐coated wells, incubated for 1 h at room temperature and then washed. All plates were exposed to an enzyme substrate consisting of 0.4 mg/ml of O‐phenylenediamine dihydrochloride (Sigma) and 0.03% v/v of hydrogen peroxide in a solution containing 0.1 M disodium hydrogen phosphate and 0.05 M citric acid monohydrate. The developed colour was read at 490 nm with a microplate reader (Bio‐Rad Lab., model 450, Richmond, CA, USA).
Analyses of IgA1 self‐aggregation using Sephacryl S‐300 column
To confirm IgA1 self‐aggregation, native and deS/deGal IgA1 was applied to a Sephacryl S‐300 column (1.5×62 cm, Pharmacia Biotech., Uppsala, Sweden) and fractionated. Inspection of each sample was performed by measuring UV absorbance at 280 nm. The relative ratio of both aggregated IgA1s in deS/deGal IgA1 and native IgA1 from the peak height was estimated (total of three points in aggregated parts/total of three points in non‐aggregated parts).
Animals
Experiments were performed with female Wistar rats weighing 200–250 g purchased from Shizuoka Jikken Doubutsu, Shizuoka, Japan.
Renal perfusion of human IgA1 in rats
The procedure used for perfusing rat kidneys was the same as that in our prior study (9).
The left kidney was exposed by a midline skin incision, under ether anaesthesia and subsequent inhalation anaesthesia (N2O 0.8 l/min, O2 0.8 l/min, Falothane 2%). The aorta was clamped above the left renal artery. A branch of the left renal artery was canulated from the aorta with polyethylene tubes (24G, Terumo, Tokyo, Japan). The left kidney was injected with 0.3 ml of saline to remove the blood and to confirm the perfused part of the kidney. Thereafter, 1 ml of PBS solution containing 2 or 5 mg of each deglycosylated IgA1 sample (native, deS, deS/deGal) was injected at an approximate flow rate of 0.2 ml/min. After the injection, the aortic clamp was removed to re‐establish normal blood flow. The rats were sacrificed at various time intervals (3, 9, 24 h) and the perfused part of the renal cortex was removed and prepared for immunofluorescence, light and electron microscopy. The number of animals examined was six rats in the native group, six in the deS and 11 in the deS/deGal groups.
Histological examination
Renal tissue was fixed with buffered formalin, embedded in parafin, and 4 μm sections were stained with periodic acid Schiff (PAS). A second portion was embedded in OCT medium (Miles Lab., Elkhart, IN, USA) immediately after sampling, frozen in liquid nitrogen and stored at −80°C until use. Frozen sections were cut to 2 μm, fixed in absolute acetone for 10 min, and then rinsed in 0.01 M phosphate buffer containing 0.015 M saline, pH 7.4 (PBS). The sections were incubated with 1/20 diluted FITC‐labelled goat anti‐human IgA antibody for 45 min at room temperature. The stained sections were rinsed with PBS and observed under a fluorescent photomicroscope (Nikon Corp., Tokyo, Japan). They were graded from 0 to 3 by the authors (TS and YH).
A third piece of renal tissue was fixed in 2% glutaraldehyde and embedded in epon for electron microscopic examination.
Results
Reactivity of PNA and VV lectins against enzymatically under‐glycosylated IgA1
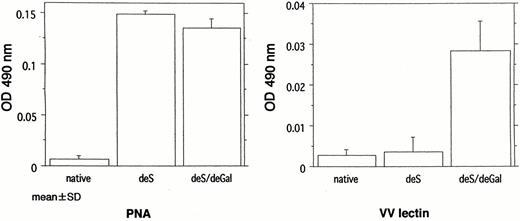
As shown in Figure 1, PNA lectin reacted with both deS and deS/deGal IgA1. VV lectin reacted with only deS/deGal IgA1. The actual levels of 490 nm absorbance in each reagent were as follows: PNA, (native) vs (deS) vs (deS/deGal); 0.007±0.004 vs 0.149±0.004 vs 0.136±0.008; VV, 0.003±0.002 vs 0.004±0.004 vs 0.028±0.007. These results indicate that the enzymatic removal of the NeuAc was appropriately carried out in deS and deS/deGal IgA1 but in deS/deGal IgA1 galactose was incompletely removed because of the reaction with PNA lectin.
Reactivity of the under‐glycosylated IgA1 with peanut agglutinin (PNA) and Vicia villosa (VV) lectin. PNA reacted with both deS IgA1 and deS/deGal IgA1. VV lectin reacted with deS/deGal IgA1.
Sephacryl S‐300 column chromatography of enzymatically under‐glycosylated IgA1
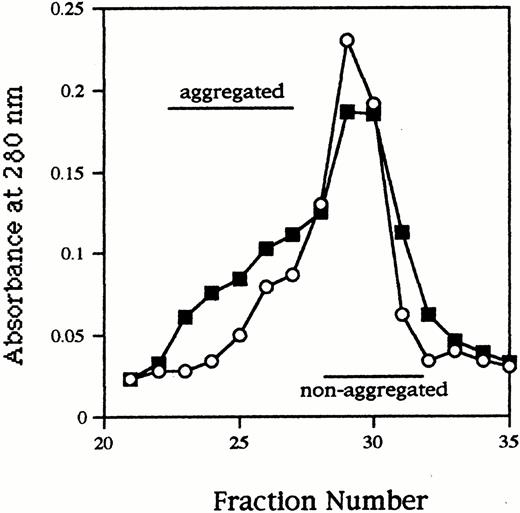
As shown in Figure 2, high molecular fractions (aggregated IgA1) were definitely increased in deS/deGal IgA1 compared to native IgA1. Obviously the ratio increased in deS/deGal IgA1 (native, 0.20; deS/deGal, 0.45).
Sephacryl S‐300 column chromatography of native IgA1 (open circles) and deS/deGal IgA1 (solid squares). High molecular fractions (aggregated IgA1) were definitely increased in deS/deGal IgA1 compared to native IgA1. The relative ratio of aggregated IgA1 in deS/deGal IgA1 was higher than native IgA1 (native; 0.20, deS/deGal; 0.45).
IgA1 accumulation in rat glomeruli and histology
Our previous study showed that mesangial deposits were present at 3 h after injection of the IgA1 fractions, and that the IgA precipitates had disappeared after 24 h. Therefore, we also sacrificed the rats at intervals of 3, 9 and 24 h.
At 3 h, as shown in Table 1, positive staining for IgA was observed in both deS IgA1 and deS/deGal IgA1 treated kidneys but no significant deposits were found in the native IgA1 treated kidneys. At 9 and 24 h these deposits had declined in the deS/deGal IgA1 injected rats.
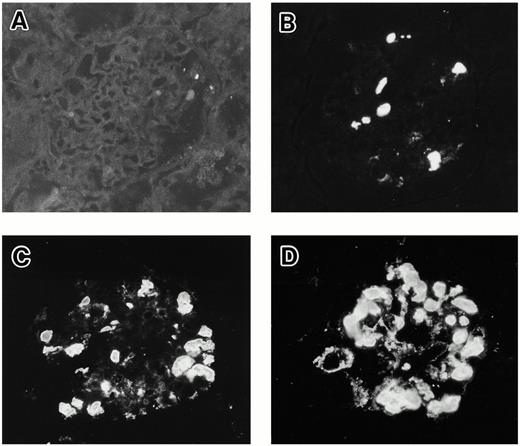
In the positive samples, IgA was accumulated in the glomerular capillary lumen and the mesangial area. Typical grading samples of immunofluorescence are shown in Figure 3.
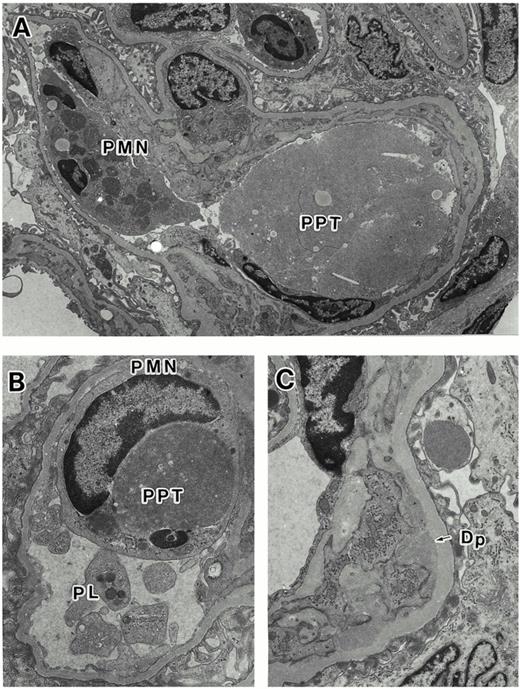
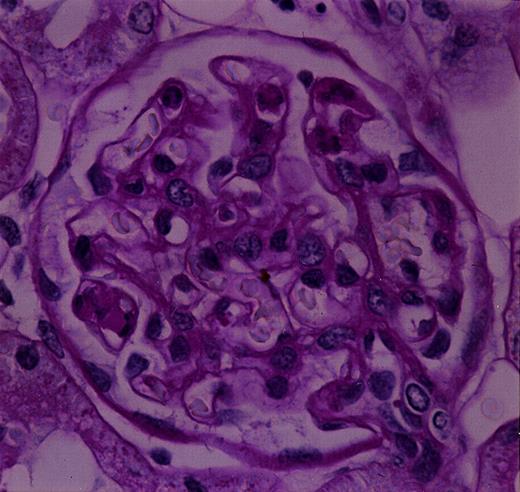
In the rats injected with both deS and deS/deGal IgA1, histological findings on electron microscopy and light microscopy showed the intraluminal precipitates (Figures 4A and 5) to correspond to the IgA precipitates observed on immunofluorescence. In addition, at 3 h, focal prominent infiltration of polymorphonuclear cells in the glomeruli were observed in deS and deS/deGal IgA1 injected rats (Figures 4A, 4B and 5). On electron microscopy, the polymorphonuclear cells in capillary lumina showed phagocytic activity against the precipitates (Figure 4B). Definite mesangial deposits could be observed on electron microscopy (Figure 4C). The severity of the deposition and infiltration was less in the rats treated with deS IgA1 than that in the rats treated with deS/deGal IgA1. However, these glomerular deposits and the inflammatory cell reaction had declined in deS/deGal IgA1 injected rats at 9 and 24 h.
Grading samples of IgA1 deposits in rat glomeruli by immunofluorescence: (A) −; (B) +; (C) ++; (D) +++ (original magnification ×350).
Histologic findings on electron microscopy. (A) The intraluminal precipitates (PPT) and the infiltration of polymorphonuclear cell (PMN) are seen (original magnification ×3790). (B) The polymorphonuclear cells in the capillary lumen showed phagocytic activity against the precipitates (original magnification ×9090). Accumulation of platelets (PL) are seen. (C) Definite mesangial deposits (Dp) are seen (original magnification ×7030).
Histologic findings on light microscopy. The intraluminal precipitates and the infiltration of polymorphonuclear cells in the glomeruli are seen (original magnification ×1635).
Accumulation of enzyme‐treated IgA1 in rat glomeruli
| Group | Dose of IgA1 (mg) | Removal time after injection (h) | Intensity of immunofluorescence | ||||||
| − | + | ++ | +++ | ||||||
| Native | 2 (n=3) | 3 | 3 | 0 | 0 | 0 | |||
| 5 (n=3) | 3 | 2 | 1 | 0 | 0 | ||||
| deS | 2 (n=3) | 3 | 0 | 3 | 0 | 0 | |||
| 5 (n=3) | 3 | 0 | 1 | 2 | 0 | ||||
| deS/deGal | 2 (n=3) | 3 | 0 | 3 | 0 | 0 | |||
| 5 (n=3) | 3 | 0 | 0 | 1 | 2 | ||||
| 5 (n=3) | 9 | 2 | 1 | 0 | 0 | ||||
| 5 (n=2) | 24 | 2 | 0 | 0 | 0 | ||||
| Group | Dose of IgA1 (mg) | Removal time after injection (h) | Intensity of immunofluorescence | ||||||
| − | + | ++ | +++ | ||||||
| Native | 2 (n=3) | 3 | 3 | 0 | 0 | 0 | |||
| 5 (n=3) | 3 | 2 | 1 | 0 | 0 | ||||
| deS | 2 (n=3) | 3 | 0 | 3 | 0 | 0 | |||
| 5 (n=3) | 3 | 0 | 1 | 2 | 0 | ||||
| deS/deGal | 2 (n=3) | 3 | 0 | 3 | 0 | 0 | |||
| 5 (n=3) | 3 | 0 | 0 | 1 | 2 | ||||
| 5 (n=3) | 9 | 2 | 1 | 0 | 0 | ||||
| 5 (n=2) | 24 | 2 | 0 | 0 | 0 | ||||
Accumulation of enzyme‐treated IgA1 in rat glomeruli
| Group | Dose of IgA1 (mg) | Removal time after injection (h) | Intensity of immunofluorescence | ||||||
| − | + | ++ | +++ | ||||||
| Native | 2 (n=3) | 3 | 3 | 0 | 0 | 0 | |||
| 5 (n=3) | 3 | 2 | 1 | 0 | 0 | ||||
| deS | 2 (n=3) | 3 | 0 | 3 | 0 | 0 | |||
| 5 (n=3) | 3 | 0 | 1 | 2 | 0 | ||||
| deS/deGal | 2 (n=3) | 3 | 0 | 3 | 0 | 0 | |||
| 5 (n=3) | 3 | 0 | 0 | 1 | 2 | ||||
| 5 (n=3) | 9 | 2 | 1 | 0 | 0 | ||||
| 5 (n=2) | 24 | 2 | 0 | 0 | 0 | ||||
| Group | Dose of IgA1 (mg) | Removal time after injection (h) | Intensity of immunofluorescence | ||||||
| − | + | ++ | +++ | ||||||
| Native | 2 (n=3) | 3 | 3 | 0 | 0 | 0 | |||
| 5 (n=3) | 3 | 2 | 1 | 0 | 0 | ||||
| deS | 2 (n=3) | 3 | 0 | 3 | 0 | 0 | |||
| 5 (n=3) | 3 | 0 | 1 | 2 | 0 | ||||
| deS/deGal | 2 (n=3) | 3 | 0 | 3 | 0 | 0 | |||
| 5 (n=3) | 3 | 0 | 0 | 1 | 2 | ||||
| 5 (n=3) | 9 | 2 | 1 | 0 | 0 | ||||
| 5 (n=2) | 24 | 2 | 0 | 0 | 0 | ||||
Discussion
In this study, we clearly observed that enzymatically under‐glycosylated IgA1 molecules were deposited in rat glomeruli. The immunofluorescence study and electron microscopic examination showed that deS and deS/deGal IgA1s were mainly accumulated in the glomerular capillary lumen and mesangial area. In addition, inflammatory cell reaction in rat glomeruli could be induced by the deposition of the under‐glycosylated IgA1.
In our most recent study, we were able to isolate and characterize the glomerulophilic IgA1, that could accumulate in rat glomeruli, from IgAN patients [9]. The glomerulophilic IgA1 molecules located in the electrically neutral fraction and the Jacalin high‐affinity fraction were clearly under‐O‐glycosylated. This is compatible with the present report where the accumulation of IgA1 in the glomeruli was observed in enzymatically under‐glycosylated IgA1.
In the previous in vitro study, we showed that IgA1 desialilated by sialidase tended to aggregate, and further degalactosylation of IgA1 by β‐galactosidase induced the adhesion of the IgA1 molecules to ECM proteins [8]. These results suggested that the aggregation and adhesion were caused simultaneously by the under‐glycosylation of the IgA1 hinge region, resulting in the glomerular deposition of the IgA1 molecules. Coppo et al. [13] also suggested that the IgA1‐ECM binding was related to the aberrant O‐glycosylation of IgA1 molecules observed in IgAN. In this study, we also observed the increase of aggregated IgA1 fractions in des/deGal IgA1, supporting the previous observations. Although, it would be reasonable to speculate that the self‐aggregation of IgA1 played a role in its glomerular deposition, the actual mechanism of the glomerular deposition of the under‐glycosylated IgA1 is still unclear.
Monteiro et al. [14] showed, in an isoelectrofocusing study, that the mesangial IgA was anionically charged. In the isoelectrofocusing study, however, another small peak (as unknown fraction) showed a relatively cationic charge. In our previous study, ‘glomerulophilic’ IgA1 was relatively cationic in the macromolecular IgA1 fraction. Furthermore, it is well known that the glomerular capillary wall and the sialic acid residue are negatively charged [15,16]. Therefore, in this study, it could be speculated that under‐glycosylation, and especially the deletion of sialic acid, influences the charge of the IgA1 molecules, resulting in an appropriate condition for IgA1 molecules to localize in glomeruli, by decreasing the electric repulsion between IgA molecules and the glomerular basement membrane.
On the other hand, it has been proposed that the binding of IgA1 to mesangial cells may be mediated through specific IgA receptors such as the asialoglycoprotein receptor and the Fcα receptor. Gomez‐Guerrero et al. [17] reported that rat and human mesangial cells possess an asialoglycoprotein receptor with specificity for the galactose residues of several glycoproteins, including human IgA1. They suggested that in situations with degalactosylated IgA1, the decreased systemic clearance of IgA1 by asialoglycoprotein receptor could facilitate their glomerular deposition. Recently, it was reported that the novel Fcα receptor expressed by human mesangial cells bound to polymeric IgA with high affinity [18]. It is possible that the aggregation of underglycosylated IgA1 directly mediates the binding to mesangial cells through both receptors.
In this study, accumulation of deS and deS/deGal IgA1s was observed in glomerular capillary lumen and the mesangial area. In addition, at 3 h after the perfusion of deS and deS/deGal IgA1, focal prominent accumulation of polymorphonuclear cells were observed in the glomerular capillary lumen. At 9 and 24 h, the glomerular deposits and the inflammatory cell reaction in the deS/deGal IgA1 injected rats had declined. These findings seem to be similar to those reported by Davin et al. [19], who also observed the histologic response induced by the perfusion of polymeric IgA–ConA complexes into the aorta of rat.
Shigematsu et al. [20] reported that, in Arthus‐type nephritis induced by the infusion of insoluble and poorly soluble immune complexes into the renal artery of rabbits, immune precipitates and accumulations of polymorphonuclear cells were observed in the capillary lumen and immune deposits were also seen in the mesangium and the subendothelial space. These deposits and the histological reaction were reduced at 24 h. This pattern of deposition and the time course of histological reaction was very similar to the findings in this study. Glomerular intraluminal deposition of under‐glycosylated IgA1 might be explained by the experimental model of insoluble and poorly soluble immune complex deposition in rabbits.
This pattern of localization of deposits was different from that of human IgA nephropathy. IgA nephropathy, clinically and histologically, is thought to undergo a slowly progressive course, as a result of a long‐term deposition of a low dose of IgA. In our experimental model, on the other hand, only a single injection of a large dose of the under‐glycosylated IgA1 was performed. This may account for the difference in the localization of deposits between our experimental model and human IgA nephropathy.
In conclusion, we were able to confirm that enzymatically under‐glycosylated IgA1 accumulated in rat glomeruli and induced an inflammatory reaction. These results indicate that under‐glycosylation of IgA1 plays an important role in the glomerular accumulation of IgA1, suggesting the existence of a similar mechanism of glomerular deposition of IgA in IgA nephropathy.
Correspondence and offprint requests to: Dr Yutaka Kobayashi, Department of Medicine, School of Medicine, Kitasato University, 1‐15‐1, Kitasato, Sagamihara‐city, Kanagawa 228‐8555, Japan. Email: ykoba@med.kitasato‐u.ac.jp
Part of this study was supported by grants from NEDO (New Energy and Industrial Technology Development Organization) and Asahi Chemical Industry. The authors thank Mr Masaki, Ms Y. Tanaka and Ms M. Saitoh for their excellent technical assistance. The authors are also grateful to the members of the Electron Microscope Laboratory Center of Kitasato University for their valuable suggestions and assistance with electron microscopy and photography.
References
Conley ME, Cooper MD, Michael AF. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis and systemic lupus erythematosus.
Coppo R, Basolo B, Martina G, Rollino C, De Marchi M, Giacchino F, Mazzucco G, Messina M, Piccoli G. Circulating immune complexes containing IgA, IgG and IgM in patients with primary IgA nephropathy and with Henoch‐Schonlein nephritis. Correlation with clinical and histologic signs of activity.
Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O‐glycosidically linked oligosaccharide units.
Hiki Y, Iwase H, Kokubo T, Horii A, Tanaka A, Nishikido J, Hotta K, Kobayashi Y. Association of asialo‐galactosylβ1–3N‐acetylgalactosamine on the hinge with a conformational instability of Jacalin‐reactive immunoglobulin A1 in immunoglobulin A nephropathy.
Iwase H, Tanaka A, Hiki Y, Kokubo T, Ishii‐Karakasa I, Kobayashi Y, Hotta K. Abundance of Galβ1,3GaNAc in O‐linked oligosaccharide on hinge region of polymerized IgA1 and heat‐aggregated IgA1 from normal human serum.
Hiki Y, Saitoh M, Kobayashi Y. Serum IgA class anti‐IgA antibody in IgA nephropathy.
Kokubo T, Hiki Y, Iwase H, Horii A, Tanaka A, Nishikido J, Hotta K, Kobayashi Y. Evidence for involvement of IgA1 hinge glycopeptide in the IgA1‐IgA1 interaction in IgA nephropathy.
Kokubo T, Hiki Y, Iwase H, Tanaka A, Toma K, Hotta K, Kobayashi Y. Protective role of IgA1 glycans against IgA1 self‐aggregation and adhesion to extracellular matrix proteins.
Hiki Y, Kokubo T, Iwase H, Masaki Y, Sano T, Tanaka A, Toma K, Hotta K, Kobayashi Y. Under‐glycosylation of IgA1 hinge plays a certain role for its glomerular deposition in IgA nephropathy.
Neurohr KJ, Young NM, Mantsch H. Determination of the carbohydrate‐binding properties of peanut agglutinin by ultraviolet difference spectroscopy.
Tollefsen S, Kornfeld R. Isolation and characterization of lectins from Vicia villosa.
Coppo R, Amore A, Gianoglio B, Reyna A, Peruzzi L, Roccatello D, Alessi D, Sena LM. Serum IgA and macromolecular IgA reacting with mesangial matrix components.
Monteiro RC, Halbwachs‐Mecarelli L, Roque‐Barreira MC, Noel LH, Berger J, Lesavre P. Charge and size of mesangial IgA in IgA nephropathy.
Kanwar YS, Farquhar MG. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes.
Rennke HG, Patel Y, Venkatachalam MA. Glomerular filtration of proteins: Clearance of anionic, neutral and cationic horseradish peroxidase in the rat.
Gómez‐Guerrero C, Duque N, Egido J. Mesangial cells possess an asialoglycoprotein receptor with affinity for human immunoglobulin A.
Barratt J, Greer MR, Pawluczyk IZA, Allen AC, Bailey EM, Buck KS, Feehally J. Identification of a novel Fcα receptor expressed by human mesangial cells.
Davin JC, Dechenne C, Mahieu PR. Acute experimental glomerulonephritis induced by the glomerular deposition of circulating polymeric IgA‐concanavalin A complexes (French).





Comments