Abstract
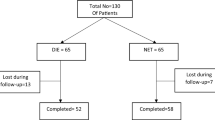
The objective of this study was to assess effectiveness and safety of Depo-Provera (medroxyprogesterone acetate) in treatment of endometrial hyperplasia (EH) and to compare it with norethisterone acetate (NETA) as an oral progestogen treatment. One hundred forty six women aged 35 to 50 years with abnormal uterine bleeding and diagnosed as having EH were randomized to receive either Depo-Provera, one injection every 3 months for 6 months (2 doses), or oral cyclic NETA, 15 mg daily for 14 days per cycle for 6 months. Primary outcome measure was regression of EH. Secondary outcome variables were side effects of treatment, persistence/progression of EH during follow-up period. After 6 months of treatment, Depo-Provera was more successful in achieving regression of nonatypical EH than NETA (67 [91.8%] of 73 women vs 49 [67.1%] of 73, respectively), and the difference between the 2 groups was statistically significant (relative risk: 1.37; 95% confidence interval: 1.15-1.63, P = .048*). Adverse effects were relatively common with moderate differences between the 2 groups. This is the first randomized study comparing Depo-Provera with an oral progestogen as a treatment for EH. Depo-Provera is an effective and safe treatment for EH without atypia.
Similar content being viewed by others
References
Epplein M, Reed SD, Voigt LF, Newton KW, Holt VL, Weiss NS. Endometrial hyperplasia risk in relation to characteristics and exposures that influence endogenous hormone levels. Am J Epidemiol. 2008; 168(6):563–570.
Reed SD, Voigt LF, Newton KM, et al. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009; 113(3):655–662.
Espíndola D, Kennedy KA, Fischer EG. Management of abnormal uterine bleeding and the pathology of endometrial hyperplasia. Obstet Gynecol Clin North Am. 2007; 34(4):717–737.
Anastasiadis PG, Skaphida PG, Koutlaki NG, Galazios GC, Tsikouras PN, Liberis VA. Descriptive epidemiology of endometrial hyperplasia in patients with abnormal uterine bleeding. Eur J Gynaecol Oncol. 2000; 21(2):131–134.
Lacey JV Jr, Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas. 2009; 63(1):39–44.
Marsden DE, Hacker NF. Optimal management of endometrial hyperplasia. Best Pract Res Clin Obstet Gynaecol. 2001; 15(3):393–405.
Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade I adenocarcinoma:a systematic review. Gynecol Oncol. 2012; 125(2):477–482.
Gultekin M, Dogan NU, Aksan G, Ozgul N. Management of endometrial hyperplasia. Minerva Ginecol. 2010; 62(5):433–445.
Eddib A, AUaf B, Lee J, Yeh J. Risk for advanced-stage endometrial cancer in surgical specimens from patients with complex endometrial hyperplasia with atypia. Gynecol Obstet Invest. 2012; 73(1):38–42.
Clark TJ, Neelakantan D, Gupta JK. The management of endometrial hyperplasia:an evaluation of current practice. Eur J Obstet Gynecol Reprod Biol. 2006; 125(2):259–264.
Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia:a Gynecologic Oncology Group study. Cancer. 2006; 106(4):812–819.
American College of Obstetricians and Gynecologists. ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005:management of endometrial cancer. Obstet Gynecol. 2005; 106(2):413–425.
Abu-Hashim H, Zayed A, Ghayaty E, El Rakhawy M. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimeno-pausal women:a randomized controlled trial. J Gynecol Oncol. 2013; 24(2):128–134.
Hom LC, Schnurrbusch U, BilekK, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia:clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer. 2004; 14(2):348–353.
Orbo A, Vereide AB, Ames M, Pettersen I, Straume B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia:a national multicentre randomised trial. BJOG. 2014; 121(4):477–486.
Bese T, Vural A, Ozturk M, et al. The effect of long-term use of progesterone therapy on proliferation and apoptosis in simple endometrial hyperplasia without atypia. Int J Gynecol Cancer. 2006; 16(2):809–813.
Westhoff C. Depot-medroxyprogesterone acetate injection (Depo-Provera):a highly effective contraceptive option with proven long-term safety. Contraception. 2003; 68(2):75–87.
Haider S, Damey F. Injectable contraception. Clin Obstet Gynecol. 2007; 50(4):898–806.
National Institute for Health and Care Excellence. Long-acting reversible contraception. NICE guidelines [CG30] October 2005. http://www.nice.org.uk/guidance/cg30/guidance/cg30. Accessed December 15, 2015.
El Behery MM, Saleh HS, Ibrahiem MA, Kamal EM, Kassem GA, Mohamed Mohamed ME. Levonorgestrel-Releasing intrauterine device versus dydrogesterone for management of endometrial hyperplasia without atypia. Reprod Sci. 2015; 22(3):329–334.
Vereide AB, Ames M, Straume B, Maltau JM, Orbo A. Nuclear morphometric changes and therapy monitoring in patients with endometrial hyperplasia:a study comparing effects of intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol. 2003; 91(3):526–533.
Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. Relapse of endometrial hyperplasia after conservative treatment:a cohort study with long-term follow-up. Hum Reprod. 2013; 28(5):1231–1236.
Buttini MJ, Jordan SJ, Webb PM. The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia:an Australian study and systematic review. Aust N ZJ Obstet Gynaecol. 2009; 49(3):316–322.
Schrager S. Abnormal uterine bleeding associated with hormonal contraception. Am Fam Physician. 2002; 65(10):2073–2081.
Haoula ZJ, Walker KF, Powell MC. Levonorgestrel intrauterine system as a treatment option for complex endometrial hyperplasia. Eur J Obstet Gynecol Reprod Biol. 2011; 159(1):176–179.
Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coo-marasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia:a systematic review and metaanalysis. Am J Obstet Gynecol. 2010; 203(6)547.e1–e10.
Demirkiran F, Yavuz E, Erenel H, Bese T, Arvas M, Sanioglu C. Which is the best technique for endometrial sampling? Aspiration (pipelle) versus dilatation and curettage (D&C). Arch Gynecol Obstet. 2012; 286(5):1277–1282.
Zaino RJ, Kauderer J, Trimble CL, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia:a Gynecologic Oncology Group study. Cancer. 2006; 106:804–811.
Gallos ID, Ofinran O, Shehmar M, Coomarasamy A, Gupta JK. Current management of endometrial hyperplasia-a survey of United Kingdom consultant gynaecologists. Eur J Obstet Gynecol Reprod Biol. 2011; 158(2):305–307.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nooh, A., Abdeldayem, H., Girbash, E.F. et al. Depo-Provera Versus Norethisterone Acetate in Management of Endometrial Hyperplasia Without Atypia. Reprod. Sci. 23, 448–454 (2016). https://doi.org/10.1177/1933719115623643
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719115623643