Abstract
Vitamin B12 is an essential micronutrient required for optimal hemopoetic, neuro-cognitive and cardiovascular function. Biochemical and clinical vitamin B12 deficiency has been demonstrated to be highly prevalent among patients with type 1 and type 2 diabetes mellitus. It presents with diverse clinical manifestations ranging from impaired memory, dementia, delirium, peripheral neuropathy, sub acute combined degeneration of the spinal cord, megaloblastic anemia and pancytopenia. This review article offers a current perspective on the physiological roles of vitamin B12, proposed pathophysiological mechanisms of vitamin B12 deficiency, screening for vitamin B12 deficiency and vitamin B12 supplementation among patients with diabetes mellitus.
Similar content being viewed by others
Introduction
Vitamin B12 or cobalamin is a water soluble vitamin that plays a very fundamental role in DNA synthesis, optimal haemopoesis and neurological function. The clinical picture of vitamin B12 deficiency hence, is predominantly of features of haematological and neuro-cognitive dysfunction [1].
This review will mainly discuss the physiological roles of vitamin B12, the varied pathophysiological mechanisms of vitamin B12 deficiency among patients with type 1 and 2 diabetes mellitus (DM) and perspectives on screening for vitamin B12 deficiency and supplementation of vitamin B12 among diabetic patients.
Absorption of vitamin B12
The principal source of vitamin B12 is animal proteins. The preliminary step in the metabolism of vitamin B12 involves its release from animal sources, a process mediated by the action of pepsin and gastric acid. After the release, dietary vitamin B12 binds to the R-protein secreted by the salivary glands. In the duodenum, in the presence of an alkaline medium and pancreatic proteases, the R- protein is hydrolysed to release vitamin B12 which later binds with the intrinsic factor (IF) secreted by the gastric parietal cells.
The vitamin B12 –IF complex is highly resistant to proteolytic degradation. The complex attaches at its specific receptors on the mucosa of the terminal ileum, a site where its absorption occurs. This stage of vitamin B12 absorption is calcium mediated.
The intracellular vitamin B12 is released following IF degradation. This free vitamin B12 attaches to another protein carrier, transcobalamin –II (TC-II) and is later released into the circulation. This vitamin B12 – TC-II complex, also referred to as holo TC-II is then actively taken up by the liver, bone marrow and other vital body cells. The liver serves as the principal storage site of up to 90% of the body’s total vitamin B12 [1, 2].
A disruption in any of the described steps above will result into clinical or biochemical vitamin B12 deficiency. This includes insufficient dietary intake especially among alcoholics and vegetarians and malabsorption due to several conditions like chronic atrophic gastritis mainly in the elderly, pernicious anemia, celiac disease, chronic pancreatitis and drugs like metformin and proton pump inhibitors (PPIs).
Physiological roles of vitamin B12
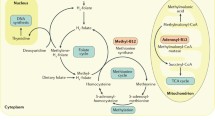
Vitamin B12 exerts its physiological effects through mediating two principal enzymatic pathways i.e. the methylation process of homocysteine to methionine and the conversion of methylmalonyl coenzyme A (CoA) to succinyl-CoA. Vitamin B12 as a co-factor facilitates the methylation of homocysteine to methionine which is later activated into S-adenosyl-methionine that donates its methyl group to methyl acceptors such as myelin, neurotransmitters and membrane phospholipids.
Metabolically significant vitamin B12 deficiency hence will result in disruption of the methylation process and accumulation of intracellular and serum homocysteine. Hyperhomocysteinemia has been shown to have potentially toxic effects on neurones and the vascular endothelium. This reaction is also essential in the conversion of dietary folate (methyl-tetrahydrofolate) to its active metabolic form, tetrahydrofolate. In another essential enzymatic pathway, vitamin B12 as a co-factor mediates the conversion of methylmalonyl coenzyme A (CoA) to succinyl-CoA. In the presence of vitamin B12 deficiency, this conversion pathway is diminished and an increase in the serum methylmalonic acid (MMA) ensues. This is followed by defective fatty acid synthesis of the neuronal membranes [3]. Vitamin B12 is also essential in the synthesis of monoamines or neurotransmitters like serotonin and dopamine [4]. This synthesis is impaired with vitamin B12 deficiency.
All the above collectively explain the resultant neuro-cognitive or psychiatric manifestations that accompany vitamin B12 deficiency. Axonal demyelination, degeneration and later death are the hallmark of vitamin B12 deficiency induced neuronal damage that manifests as severe peripheral or autonomic neuropathy, sub acute combined degeneration of the spinal cord, delirium and dementia [3, 5]. Compelling evidence demonstrates that hyperhomocysteinemia is also associated with an increased risk of cardiovascular events due to its cellular and vasculo-toxic effects [6–8].
Vitamin B12 is an essential micronutrient required in DNA synthesis, cellular repair and normal haemopoesis together with other micronutrients like folate and iron. Vitamin B12 deficiency is classically associated with overt haematological findings like macrocytic red blood cells (mean cell volume [MCV]> 100 fl) with/without anaemia, ovalocytes, hyper segmented white blood cells (i.e. >5% of neutrophils with ≥5 lobes) and pancytopenia [9]. Due to defective cell repair processes, atrophic glossitis, stomatitis and malabsorption due to villi atrophy and mucositis are also common.
Vitamin B12 deficiency among patients with type 2 diabetes mellitus and the general population: a comparative review
Several cross sectional studies [10–12] and case reports [13–15] have documented an increased frequency of vitamin B12 deficiency among type 2 DM (T2DM) patients. Metformin use has been unequivocally demonstrated as the prime factor associated with vitamin B12 deficiency among patients with T2DM [16–19]. Studies assessing type 2 diabetic patients on metformin have reported the prevalence of vitamin B12 deficiency to range from 5.8% to 33% [10, 19, 20]. This wide variation in the reported prevalence could probably be explained by the varied study definitions of vitamin B12 deficiency. In the cross sectional study by Pflipsen et al. on 203 outpatient type 2 diabetic patients at a large military primary care clinic in USA, definite vitamin B12 deficiency was defined as serum vitamin B12 concentrations of <100 pg/ml or elevated serum methylmalonic acid of >243 nmol/L or homocysteine concentrations of >11.9 nmol/L if serum vitamin B12 concentrations were between 100 to 350 pg/mL [10]. Reinstatler et al. in the National Health and Nutrition Examination Survey of 1999–2006 in the USA defined definite and borderline biochemical vitamin B12 deficiency as serum vitamin B12 concentrations of ≤148 pmol/l and >148-221 pmol/l respectively [19]. In one cross sectional study that documented a high prevalence of vitamin B12 deficiency of 33% among adult patients with T2DM by Qureshi et al., vitamin B12 deficiency was defined as serum vitamin B12 concentrations <150 pg/ml [20]. However, patients enrolled in this study were those who were on high dose (>2 g/day) and long-term (4 years) metformin treatment, both clinical factors known to be associated with vitamin B12 deficiency [17, 18].
Due to the diverse definitions of vitamin B12 deficiency used in most studies and the cultural and religious beliefs in different regions of the world, comparison of the prevalence of vitamin B12 deficiency among T2DM patients and healthy general populations is difficult. In one population based study among 1048 elderly Finnish subjects aged 65–100 years, the total prevalence of definite vitamin B12 deficiency was 12.1% [21]. Previously diagnosed vitamin B12 deficiency was reported among 2.6% of the participants. Vitamin B12 replacement therapy was documented among only 2.6% of the participants. In this study, vitamin B12 deficiency was defined as total serum vitamin B12 concentrations < 150 pmol/l or total serum vitamin B12of 150–250 pmol/l and holotranscobalamin ≤37 pmol/l and homocysteine ≥15 μmol/l.
In India, a country with a large proportion of vegetarians due to cultural and religious beliefs, very high prevalence of vitamin B12 deficiency among the general population has been reported. In one study by Yajnik et al. to determine the frequency of vitamin B12 deficiency and hyperhomocysteinemia among 441 healthy middle aged Indian men, vitamin B12 deficiency as defined by vitamin B12 concentrations <150 pmol/L was reported among 67% of the study participants [22]. Vegetarian diet was the sole significant factor associated with low vitamin B12 levels in this study on multivariate analysis (OR 4.4 95% CI 2.1-9.3).
In another cross sectional study among 175 healthy elderly Indian subjects aged >60 years, vitamin B12 deficiency also defined as vitamin B12 concentrations <150 pmol/L was reported among 16% of the study participants [23]. Elevated serum MMA concentrations which are a more sensitive indicator of vitamin B12 deficiency were documented among 55% of the participants.
Metformin induced vitamin B12 deficiency among patients with T2DM
In the absence of contraindications like renal and hepatic dysfunction, recent guidelines advocate for the use of metformin as the first line glucose lowering agent concurrently with life style modification approaches [24, 25]. Despite its very superior glycemic lowering effect, metformin has for long been shown to decrease vitamin B12 levels. In one early randomised controlled trial by DeFronzo et al., metformin decreased the serum vitamin B12 levels by 22% and 29% compared to placebo and glyburide respectively [26]. This side effect of metformin has been demonstrated again in several ensuing cross sectional studies [10–12], case reports [13–15] and randomised controlled trials [16, 17].
The risk of developing metformin associated vitamin B12 deficiency is greatly influenced by increasing age, metformin dose and duration of use [17, 18]. In a nested case control study performed among 155 adult Chinese DM patients on metformin and 310 controls, every 1 g/day increase in the metformin dose conferred an odds ratio of 2.9 (95% confidence interval, 2.15-3.87) for developing vitamin B12 deficiency. Among patients using metformin for ≥ 3 years, the adjusted odds ratio was 2.4 (95% confidence interval, 1.46-3.91) compared with those who had received metformin for ≤ 3 years [18].
Decrease in vitamin B12 absorption and levels following metformin use typically starts as early as the 4th month [27]. Clinically overt features of vitamin B12 deficiency manifest by 5–10 years owing to the large body stores in the liver mainly that are not quickly depleted [28].
The proposed mechanisms to explain metformin induced vitamin B12 deficiency among patients with T2DM include: alterations in small bowel motility which stimulates bacterial overgrowth and consequential vitamin B12 deficiency, competitive inhibition or inactivation of vitamin B12 absorption, alterations in intrinsic factor (IF) levels and interaction with the cubulin endocytic receptor [28]. Metformin has also been shown to inhibit the calcium dependent absorption of the vitamin B12-IF complex at the terminal ileum. This inhibitory effect is reversed with calcium supplementation [29].
Vitamin B12 deficiency among patients with type 1 diabetes mellitus
Type 1 DM (T1DM) is an auto immune condition that results from auto immune destruction of insulin secreting beta cells of the pancreas. It is invariably associated with other organ and non organ specific auto immune and endocrine conditions leading to development of autoimmune polyglandular syndromes [30].
Pernicious anemia resulting from chronic autoimmune gastritis is highly frequent among patients with T1DM. Chronic autoimmune gastritis and pernicious anemia occurs in about 2% and up to 1% of the general population respectively. Among patients with T1DM, the prevalence is increased by 3 to 5 folds [31].
Vitamin B12 deficiency due to pernicious anemia occurs frequently among patients with T1DM. In one cross sectional study done in South India among 90 patients with T1DM, low vitamin B12 levels were noted among 45.5% of the study subjects as defined by the manufactures’ cut off point of <180 pg/ml and among 54% using the published cut off point of <200 pg/ml [32]. No positive correlation was noted between low vitamin B12 levels and gender, age, duration of DM and level of glycemic control.
Patients with T1DM actively exhibit auto antibodies to intrinsic factor (AIF) type 1 and 2 [31] and parietal cell antibodies (PCA) [33, 34] especially those with glutamate decarboxylase-65 (GAD-65) antibodies and HLA-DQA1*0501-B1*0301 haplotype [35].
The PCA inhibit secretion of intrinsic factor resulting into pernicious anemia, a condition which is 10 times more prevalent among type 1 diabetic patients than non diabetic patients. Type 1 AIF result into vitamin B12 deficiency by blocking the binding of vitamin B12 to IF. This prevents its transportation to its absorption site, the terminal ileum. These auto antibodies are found in 70% of patients with pernicious anemia [31].
Primary autoimmune hypothyroidism and celiac disease are frequent co morbidities among patients with T1DM [36–38] and directly affect vitamin B12 metabolism [39, 40]. In one cross sectional study among 504 ambulatory T1DM patients in South Africa, the overall prevalence of co-existing auto immune hypothyroidism was 20.2%, especially among female patients (30.9% Vs 10.1%-males, p<0.0002). Celiac disease in this study cohort was reported in 3 (0.6%) patients [37].
Vitamin B12 deficiency among patients with autoimmune hypothyroidism could be explained by the presence of antibodies to the gastric parietal cells and intrinsic factor, reduced oral intake, dyserythropoesis due to thyroid hormone deficiency and defective absorption due to reduced bowel motility, bowel wall oedema and bacterial overgrowth [40].
Celiac disease which is a highly prevalent autoimmune mediated gastrointestinal condition occurs in 1-16% of T1DM patients compared to 0.3-1% in the general population [36]. Ingestion of wheat gluten and other related proteins have been documented to be the trigger factors of this condition in genetically susceptible individuals. Due to the associated enteropathy, patients often present with failure to thrive, chronic diarrhea and anemia due to micronutrient (mainly folate, vitamin B12) malabsorption [41].
Screening approach for vitamin B12 deficiency among patients with T2DM
Currently, there are no published guidelines advocating for routine screening for vitamin B12 deficiency among patients with T2DM. However among type 2 diabetic patients, it is clinically plausible to screen for vitamin B12 deficiency prior to initiation of metformin and later annually among elderly patients with history of long term use of metformin (≥3-4 years), use of high doses of metformin (≥2 g/day), clinically worsening diabetic distal polyneuropathy in the presence or absence of the discussed haematological abnormalities [42].
The screening approach for vitamin B12 deficiency among diabetic patients and the general population is similar. Measurement of the serum vitamin B12 concentrations should be the preliminary screening step for vitamin B12 deficiency among patients with T2DM. Concentrations <200 pg/ml are usually diagnostic of vitamin B12 deficiency while concentrations >400 pg/ml confirm absence of vitamin B12 deficiency [43].
Measurement of serum MMA or homocysteine concentrations is a more sensitive and specific approach for screening especially among type 2 diabetic patients with borderline serum vitamin B12 concentrations of 200-400 pg/ml and subtle haematological manifestations. Serum homocysteine and MMA concentrations of 5-15 μmol/l and <0.28 μmol/l are considered within the normal range respectively [42, 44].
Screening for vitamin B12 deficiency among patients with T1DM
Among patients with T1DM, there are no clear guidelines regarding screening for vitamin B12 deficiency. However, due to the high prevalence of pernicious anaemia and subsequent vitamin B12 deficiency among T1DM patients reported in most cross sectional studies, it would be pragmatic to screen at diagnosis and then later yearly for 3 years, then five yearly thereafter or in presence of any clinical indication since vitamin B12 deficiency can develop at anytime [31]. Screening should involve assessment of serum vitamin B12 levels and markers of gastric autoimmunity like PCA and AIF especially among T1DM patients with GAD-65 and thyroid peroxidase antibodies. Presence of these auto antibodies increases the propensity to developing vitamin B12 deficiency [31, 45].
Treatment of vitamin B12 deficiency among diabetic patients
Treatment of vitamin B12 deficiency does not differ regardless of the aetiology. All patients deficient of vitamin B12 should receive replacement therapy with either oral or parenteral vitamin B12 [46, 47]. Both formulations have been demonstrated to induce comparable desirable haematological and neurological improvements regardless of the aetiology of the deficiency [48]. In adult patients with T2DM, intra muscular or oral vitamin B12 in doses of 1000 μg daily for a week then once every week for 4 weeks are sufficient to correct vitamin B12 deficiency [46, 47].
Among young patients with T1DM and co-existing vitamin B12 deficiency, replacement therapy with daily intra muscular or oral vitamin B12 in the dose of 100μg for a week and then monthly is satisfactory. In severe cases, parenteral or oral administration of 1000 μg/day of vitamin B12 for a week, followed by the similar dose given every week for 1 month and then later monthly is advised [31].
Concomitant folate deficiency should be treated with oral folate replacement in doses of 5 mg daily for 1–4 months. Folate administration prior to correcting vitamin B12 deficiency should be avoided because it results into progression and / worsening of the associated neurological manifestations [46].
Therapeutic benefits of vitamin B12 replacement among T2DM patients with diabetic neuropathy
Vitamin B12 deficiency and the accompanying hyperhomocysteinemia and elevated MMA levels have been documented to cause a distinct sensory polyneuropathy that closely mimics diabetic neuropathy. Worsening of diabetic neuropathy is also noted among patients with co-existing vitamin B12 deficiency [49].
Vitamin B12 replacement has been shown to cause symptomatic improvement among patients with severe diabetic neuropathy. One meta-analysis showed that if used either alone or in combination with vitamin B complex, there was a significant improvement in the somatic symptoms like pain and paraesthesias. Three included studies also noted an improvement in autonomic symptoms with use of vitamin B12 alone [50].
Similar superior positive findings of reduction in pain and paraesthesias were also noted with use of vitamin B12 as compared to nortriptyline in a randomized, single-blind clinical trial done in Iran among 100 patients with diabetic neuropathy. This study was approved by the Ethics review board of the Isfahan University of Medical Sciences, Iran [51].
Vitamin B12 supplementation among patients with DM
There are no guidelines to address how often patients with T1DM and T2DM should be supplemented with vitamin B12. The optimal supplementation dose of vitamin B12 is also unknown. A recently published follow-up study from the United States of America showed that administration of oral vitamin B12 among type 2 DM patients on long term use of metformin was ineffective in correcting biochemical vitamin B12 deficiency19. The doses of vitamin B12 in the multivitamin formulations used by the study subjects in this survey were probably inadequate to correct vitamin B12 deficiency.
This stresses the need of further studies to determine the optimal vitamin B12 supplementation dose and frequency of supplementation among patients with DM. To avert vitamin B12 deficiency especially among adult type 2 diabetic patients on long term use of metformin, it is plausible to adopt a simple and cost effective supplementation approach in diabetes care. A 1000 μg dose of vitamin B12 given annually would be sufficient to replenish the body’s vitamin B12 stores among this category of patients [52].
Conclusions
Clinical and biochemical vitamin B12 deficiency is highly prevalent among patients with both types 1 and 2 DM. Future large and well designed studies on screening for vitamin B12 deficiency, vitamin B12 supplementation and optimal supplementation dose among type 1 and type 2 diabetic patients are warranted to help guide formulation of guidelines in diabetes clinical care. Annual screening for vitamin B12 deficiency using more sensitive methods like serum homocysteine and methylmalonic acid concentrations (in clinical settings where they are accessible) and supplementation should be adopted among diabetic patients with specific risk factors of vitamin B12 deficiency.
References
Oh R, Brown D: Vitamin B12 Deficiency. Am Fam Physician 2003, 67: 979–86.
Andrès E, Loukili N, Noel E: Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004, 171: 251–9. 10.1503/cmaj.1031155
Malouf R, Areosa S: Vitamin B12 for cognition. Cochrane Database of Systematic Reviews 2003.
Bottiglieri T, Laundy M, Crellin R: Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry 2000, 69: 228–32. 10.1136/jnnp.69.2.228
Selhub J, Morris M, Jacques P, Rosenberg I: Folate–vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr 2009, 89: 702S-6S. 10.3945/ajcn.2008.26947C
Melhem A, Desai A, Hofmann M: Acute myocardial infarction and pulmonary embolism in a young man with pernicious anemia-induced severe hyperhomocysteinemia. Thromb J 2009, 7: 5. 10.1186/1477-9560-7-5
Selhub J: Public health significance of elevated homocysteine. Food Nutr Bull 2008, 29: S116–25.
Sadeghian S, Fallahi F, Salarifar M: Homocysteine, vitamin B12 and folate levels in premature coronary artery disease. BMC Cardiovasc Disord 2006, 6: 38. 10.1186/1471-2261-6-38
Aslinia F, Mazza J, Yale S: Megaloblastic Anemia and Other Causes of Macrocytosis. Clinical Medicine & Research 2006, 4: 236–41. 10.3121/cmr.4.3.236
Pflipsen M, Oh R, Saguil A, Seehusen D, Topolski R: The Prevalence of Vitamin B12 Deficiency in Patients with Type 2 Diabetes: A Cross-Sectional Study. J Am Board Fam Med 2009, 22: 528–34. 10.3122/jabfm.2009.05.090044
Hermann L, Nilsson B, Wettre S: Vitamin B12 status of patients treated with metformin: a cross-sectional cohort study. British Journal of Diabetes & Vascular Disease 2004, 4: 401. 10.1177/14746514040040060701
Nervo M, Lubini A, Raimundo F: Vitamin B12 in metformin-treated diabetic patients: a cross-sectional study in Brazil. Rev Assoc Med Bras 2011, 57: 46–9.
Liu K, Dai L, Jean W: Metformin-related vitamin B12 deficiency. Age Ageing 2006, 35: 200–1. 10.1093/ageing/afj042
Bell D: Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathy. South Med J 2010, 103: 265–7. 10.1097/SMJ.0b013e3181ce0e4d
Kumthekar A, Gidwani H, Kumthekar A: Metformin Associated B12 Deficiency. Journal of the Association of Physicians of India 2012, 60: 58–9.
Kos E, Liszek M, Emanuele M, Durazo-Arvizu R, Camacho P: Effect of metformin therapy on vitamin D and vitamin B12 levels in patients with type 2 diabetes mellitus. Endocr Pract 2012, 18: 179–84. 10.4158/EP11009.OR
De-Jager J, Kooy A, Lehert P: Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 2010, 340: c2181. 10.1136/bmj.c2181
Ting R, Szeto C, Chan M, Ma K, Chow K: Risk Factors of Vitamin B12 Deficiency in Patients Receiving Metformin. Arch Intern Med 2006, 166: 1975–9. 10.1001/archinte.166.18.1975
Reinstatler L, Qi Y, Williamson R, Garn J, Oakley-Jr G: Association of Biochemical B12 Deficiency With Metformin Therapy and Vitamin B12 Supplements. The National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care 2012, 35: 327–33. 10.2337/dc11-1582
Qureshi S, Ainsworth A, Winocour P: Metformin therapy and assessment for vitamin B12 deficiency: is it necessary? Practical Diabetes 2011, 28: 302–4. 10.1002/pdi.1619
Loikas S, Koskinen P, Irjala K: Vitamin B12 deficiency in the aged: a population-based study. Age and Ageing 2007, 36: 177–83. 10.1093/ageing/afl150
Yajnik C, Deshpande S, Lubree H: Vitamin B12 Deficiency and Hyperhomocysteinemia in Rural and Urban Indians. JAPI 2006, 54: 775–82.
Shobhaa V, Tareya S, Singh R: Vitamin B12 deficiency and levels of metabolites in an apparently normal urban south Indian elderly population. Indian J Med Res 2011, 134: 432–9.
Day C: ADA-EASD diabetes guidance: individualised treatment of hyperglycaemia. British Journal of Diabetes & Vascular Disease 2012, 12: 146. 10.1177/1474651412450477
American Diabetes Association Position statement- Standards of Medical Care in Diabetes. Diabetes Care 2012, 35: S11-S63.
DeFronzo R, Goodman A: Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 1995, 333: 541–9. 10.1056/NEJM199508313330902
Wulffele M, Kooy A, Lehert P: Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med 2003, 254: 455–63. 10.1046/j.1365-2796.2003.01213.x
Andre`s E, Noel E, Goichot B: Metformin-associated vitamin B12 deficiency. Arch Intern Med 2002,162(Andre`s E, Noel E, Goichot B):2251–2.
Bauman W, Shaw S, Jayatilleke E, Spungen A, Herbert V: Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 2000, 23: 1227–31. 10.2337/diacare.23.9.1227
Van-den-Driessche A, Eenkhoorn V, Van-Gaal L, DeBlock C: Type 1 diabetes and autoimmune polyglandular syndrome: a clinical review. The Netherlands journal of Medicine 2009, 67: 376–87.
De-Block C, De-Leeuw H, Van-Gaal L: Autoimmune Gastritis in Type 1 Diabetes: A Clinically Oriented Review. J Clin Endocrinol Metab 2008, 93: 363–71. 10.1210/jc.2007-2134
Koshy A, Kumari J, Ayyar V, Kumar P: Evaluation of serum vitamin B12 levels in type 1 diabetics attending a tertiary care hospital: a preliminary cross sectional study. Indian Journal of Endocrinology and Metabolism 2012, 16: S79–82. 10.4103/2230-8210.94270
Riley W, Toskes P, Maclaren N, Silverstein J: Predictive value of gastric parietal cell autoantibodies as a marker for gastric and hematologic abnormalities associated with insulin-dependent diabetes. Diabetes 1982, 31: 1051–5. 10.2337/diabetes.31.12.1051
De-Block C, Van-Gaal L, De-Leeuw I, the-Belgian-Diabetes-Registry: High prevalence of manifestations of gastric autoimmunity in parietal cell-antibody positive type 1 (insulin-dependent) diabetic patients. J Clin Endocrinol Metab 1999, 84: 4062–7. 10.1210/jc.84.11.4062
De-Block C, De-Leeuw I, Rooman R, Winnock F, Du-Caju M, Van-Gaal L: Gastric parietal cell antibodies are associated with glutamic acid decarboxylase-65 antibodies and the HLA DQA1*0501–DQB1*0301 haplotype in type 1 diabetes. Diabet Med 2000, 17: 618–22. 10.1046/j.1464-5491.2000.00354.x
Rewers M, Liu E, Simmons J, Redondo M, Hoffenberg E: Celiac disease associated with type 1 diabetes mellitus. Endocrinol Metab Clin North Am 2004, 33: 197–214. 10.1016/j.ecl.2003.12.007
Joffe B, Distiller L, Landau S, Blacking L, Klisiewicz A: Spectrum of Autoimmune Disorders in Type 1 Diabetes – A Cross-Sectional Clinical Audit. J Diabetes Metab 2010, 1: 112.
Barker J: Type 1 Diabetes-Associated Autoimmunity: Natural History, Genetic Associations, and Screening. J Clin Endocrinol Metab 2006, 91: 1210–7. 10.1210/jc.2005-1679
Roberts C, Ladenson P: Hypothyroidism. Lancet 2004, 363: 793–803. 10.1016/S0140-6736(04)15696-1
Fein H, Rivlin R: Anemia in thyroid diseases. Medical Clinics of North America 1975, 59: 1133–45.
Selimog˘lu M, Karabiber H: Celiac Disease- Prevention and Treatment. J Clin Gastroenterol 2010, 44: 4–8. 10.1097/MCG.0b013e3181b7ead2
Mazokopakis E, Starakis I: Recommendations for diagnosis and management of metformin-induced vitamin B12 (Cbl) deficiency. Diabetes research and clinical practice 2012, 97: 359–67. 10.1016/j.diabres.2012.06.001
Snow C: Laboratory Diagnosis of vitamin B12 and folate deficiency. A guide for the primary care physician. Arch Intern Med 1999, 159: 1289–98. 10.1001/archinte.159.12.1289
Klee G: Cobalamin and folate evaluation: measurements of methylmalonic acid and homocystein vs vitamin B12 and folate. Clin Chem 2000, 46: 1277–83.
Eisenbarth G, Gottlieb P: Autoimmune Polyendocrine Syndromes. N Engl J Med 2004, 350: 2068–79. 10.1056/NEJMra030158
Hvas A, Nexo E: Diagnosis and treatment of vitamin B12 deficiency. An update. Haematologica 2006, 91: 1506–12.
Andres E, Serraj K: Optimal management of pernicious anemia. Journal of Blood Medicine 2012, 3: 97–103.
Butler C, Vidal-Alaball J, Cannings-John R: Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract 2006, 10: 279–85.
Wile D, Toth C: Association ofmetformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care 2010, 33: 156–61. 10.2337/dc09-0606
Sun Y, Lai M, Lu C: Effectiveness of Vitamin B12 on Diabetic Neuropathy: Systematic Review of Clinical Controlled Trials. Acta Neurol Taiwan 2005, 14: 48–54.
Talaei A, Siavash M, Majidi H, Chehrei A: Vitamin B12 may be more effective than nortriptyline in improving painful diabetic neuropathy. Int J Food Sci Nutr 2009, 60: 71–6. 10.1080/09637480802406153
Mahajan R, Gupta K: Revisiting metformin: annual vitamin B12 supplementation may become mandatory with longterm metformin use. J Young Pharm 2010, 2: 428–9. 10.4103/0975-1483.71621
Acknowledgements
We acknowledge the entire medical team of the department of Medicine, St. Raphael of St. Francis hospital Nsambya, Uganda.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare no competing interests.
Authors’ contributions
Both authors equally contributed to the development of the concept and manuscript, critically read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kibirige, D., Mwebaze, R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified?. J Diabetes Metab Disord 12, 17 (2013). https://doi.org/10.1186/2251-6581-12-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-12-17