Article Text
Abstract
Objective We sought to evaluate prognostic markers of clinical outcome in asymptomatic patients with moderate to severe aortic stenosis (AS).
Design Prospective follow-up of asymptomatic patients with moderate to severe AS. The patients underwent clinical and Doppler echocardiographic evaluation.
Setting Department of Cardiology.
Patients 163 patients with moderate to severe AS (aortic valve area ≤0.6 cm2/m2).
Main outcome measures Risk stratification. Predefined endpoints for assessing the outcome were the occurrence during follow-up of symptoms, aortic valve replacement or death.
Results During follow-up (mean, 20 (19) months), 11 patients developed symptoms but were not operated on, 57 required aortic valve replacement and six patients died. In multivariable Cox regression analysis, four parameters that were associated with the outcome were identified: peak aortic jet velocity, left ventricular systolic (LV) longitudinal deformation, valvulo-arterial impedance and indexed left atrial area. Using receiver−operator characteristic curve analysis, a peak aortic jet velocity ≥4.4 m/s, a LV longitudinal myocardial deformation ≤15.9%, a valvular-arterial impedance ≥4.9 mm Hg/ml per m2 and an indexed left atrial area ≥12.2 cm2/m2 were identified as the best cut-off values to be associated with events.
Conclusions In asymptomatic patients with moderate to severe AS, measurements that integrate the ventricular, vascular and valvular components of the disease improve risk stratification.
- Aortic stenosis
- echocardiography
- left ventricular function
- exercise
- valve surgery
- aortic value disease
Statistics from Altmetric.com
Aortic stenosis (AS) is the most common valvular disease in developed countries. Its prevalence is expected to markedly increase as the population ages. Patients with symptoms with severe AS have a high mortality rate and require prompt aortic valve replacement (AVR).1 Although asymptomatic patients are at increased risk for untoward events, their management remains controversial.2 3 Current guidelines consider surgery as reasonable in asymptomatic patients with reduced (<50%) left ventricular (LV) ejection fraction and in patients who exhibit symptoms during an exercise test.4 5 Improving the identification of patients at high risk of cardiac events is a challenging issue.
We hypothetised that measurements integrating valvular, vascular and ventricular components of the disease could identify new markers of guarded prognosis and allow a better timing of intervention. To test this hypothesis, we prospectively studied consecutive patients with asymptomatic moderate to severe AS who did not fulfil current criteria of reasonable surgical option at the time of inclusion.
Methods
Patient population
A total of 163 asymptomatic patients with significant AS were enrolled between January 2000 and December 2007 in this prospective study. All patients met the following specific criteria: moderate to severe AS defined by an aortic valve area ≤0.6 cm2/m2, absence of symptoms according to a careful history taking by both the referring physician and us, normal LV ejection fraction (≥55%) as calculated by two-dimensional echocardiography, no more than mild associated cardiac valve lesion and sinus rhythm. At study entry, the following clinical data were collected: age, gender, history of hypercholesterolemia (total cholesterol >190 mg/dl or patients under lipid lowering therapy), current smoking, diabetes mellitus, systemic arterial hypertension (blood pressure ≥ 140/90 mm Hg or patients under anti-hypertensive treatment) and overweight (body mass index >25 kg/m2). The protocol was approved by the relevant institutional review boards and all patients gave written informed consent.
Echocardiographic measurements
Doppler echocardiographic examinations were performed with the use of a VIVID 7 ultrasound system (General Electric Healthcare, Little Chalfont, UK). M-mode, two-dimensional, colour Doppler and pulsed-wave and continuous-wave Doppler data were stored on a dedicated workstation for off-line analysis. For each measurement, at least two cardiac cycles were averaged. Continuous wave Doppler was used to measure the aortic transvalvular maximal velocities; peak and mean gradients were calculated using the simplified Bernoulli equation. Aortic valve area was calculated using the continuity equation.6 Stroke volume was calculated using the Doppler method as follows: 0.785 × (LV outflow tract diameter)2 × LV outflow tract velocity time integral. LV end-diastolic and end-systolic volumes and ejection fraction were measured by the bi-apical Simpson disk method.7 Left atrial area was obtained by planimetry of an end-systolic frame from the apical four-chamber view.8 Peak E-wave and A-wave velocities of the mitral inflow were measured using pulsed wave Doppler. To complete the analysis of the LV systolic function, the global longitudinal myocardial deformation was evaluated from standard two-dimensional images (frame rates ≥60/s). Two-dimensional strain is a non-Doppler-based method. In brief, by tracing the endocardial borders on an end-systolic frame, the software automatically tracked the contour on the subsequent frames. Adequate tracking was verified in real-time and was manually corrected, if necessary. The global longitudinal deformation—strain—was the average of the segment strains from the apical four-chamber and two-chamber views.9 The peak velocities of the E wave (early diastole) and the A wave (late diastole) were measured and the ratio of these velocities was calculated.
Systemic arterial haemodynamics and global LV afterload
Systemic arterial pressure was measured with the use of an arm-cuff sphygmomanometer at the time of the Doppler echocardiographic examination. The ratio of the stroke volume index to the brachial pulse pressure (the difference between the systolic and the diastolic blood pressure) was used as an indirect measure of the total systemic arterial compliance. To estimate the global LV afterload, we calculated the valvulo-arterial impedance as the sum of the systolic arterial pressure and the mean transvalvular pressure gradient divided by the stroke volume index.10
Exercise testing
Symptom-limited graded bicycle exercise tests were performed at inclusion in all patients. After an initial workload of 25 W maintained for 2 min, the load was increased by steps of 25 W every 2 min. A 12-lead ECG was monitored continuously. The exercise test was interrupted when age-related maximum heart rate was reached or, in case of symptoms (angina, dyspnea), fall in blood pressure or ventricular arrhythmias. The test was considered abnormal if the patient presented ≥1 of the following criteria: angina, evidence of dyspnea, dizziness, syncope or near-syncope, ≥2 mm ST segment depression in comparison to baseline levels, rise in systolic blood during exercise <20 mm Hg or a fall in blood pressure and complex ventricular arrhythmias.
Follow-up
Follow-up information was obtained from interviews with the patients, their relatives or their physicians every six to 12 months, according to guidelines.4 5 Particular care was taken to obtain information regarding the development of symptoms, the eventual AVR and death. The clinical management of the patients was determined independently by their personal physicians.
Statistical analysis
Data are expressed as mean±SD or percentages unless otherwise specified. Group comparisons were obtained for categorical variables with χ2 test and for continuous variables with one-way analysis of variance. The combined end-point of the study included: the development of significant symptoms (angina, dyspnea, syncope, heart failure), cardiac death and the clinical need of AVR. The analysis was performed by censoring follow-up at the time of cardiac surgery if eventually performed. Multivariate Cox proportional-hazards regression procedure was used to identify independent predictors of events (Statistica Software, V.7). Clinically relevant variables with a p value <0.1 on univariable analysis were incorporated into the multivariable models. Variables were used in the model as continuous or categorical variables when possible. The Kaplan–Meier method was used for cumulative survival analysis with the log-rank test for assessing statistical differences between curves. A p value <0.05 was considered to indicate statistical significance. Receiver–operator characteristic curves were generated to determine the performance of independent variables for the prediction of cardiac events. An additional analysis was performed using multiple logistic regression to identify the independent predictor of 1-year event-free survival. Patients with less than 1-year follow-up were excluded from this analysis.
Results
Characteristics of the patients
Based on the patient's history and the echocardiographic analysis, the origin of AS was calcific in 148 patients (91%) and bicuspid in 15 (9%). The aortic valve area ranged from 0.2 to 0.6 cm2/m2 and the mean transaortic pressure gradient ranged from 25 to 86 mm Hg. Mean LV ejection fraction was 67 (7)% (55–84%). A history of systemic arterial hypertension was noted in 81 patients; 72 patients had hypercholesterolemia and 27 patients were diabetic. At inclusion, the exercise test was abnormal (dyspnea, n=25; angina=3; both symptoms, n=2; fall or rise in systolic blood during exercise <20 mm Hg, n=15; ≥2 mm ST segment depression as compared with rest, n=13; combined parameters, n=11) in 69 (42%) patients.
Clinical outcome
Follow-up information was available in all 163 patients. The mean follow-up time was 20±19 months (range 4–102 months) (figure 1). During follow-up, predefined end-points were reached in 74 patients including six deaths, 57 patients who required AVR and 11 patients who developed symptoms but did not have valve replacement (three refused surgery and eight were on the waiting list at the time of follow-up). Six patients presented a death due to cardiac causes (three to congestive heart failure related to AS and three sudden deaths without preceding symptoms). One additional patient died postoperatively from endocarditis. Another patient died from cancer. AVR was required by the development of symptoms in 44 patients within 15 (13) months following inclusion. The predominant symptoms were severe dyspnea, angina, or syncope in 26, six and three patients respectively. Nine patients developed both angina and dyspnea. In the remaining 13 patients, surgery was performed because of the onset of severely symptomatic atrial fibrillation (n=1), a newly positive exercise test during follow-up (n=6) or equivocal symptoms (n=6). Eighty-nine patients remained free of clinical event after a follow-up of 26±22 months.
Kaplan–Meier event-free survival curve of the whole cohort (n=163). The mean±SD survival rates at 2 and 4 years are indicated.
Predictors of events
The clinical and echocardiographic characteristics of the patients who remained asymptomatic and those who experienced an event are listed in table 1. No clinical parameters (age, sex, risk factors, blood pressure) allowed significant distinction between the two groups. By contrast, patients who developed events had markedly reduced aortic valve area and systemic arterial compliance and had significantly higher valvulo-arterial impedance and larger left atrium than those remaining asymptomatic. Although conventional indices of global LV systolic function (LV volumes and ejection fraction) were statistically similar in the two groups, the LV longitudinal myocardial deformation was impaired in the group of patients who developed an event. The response to exercise was more often abnormal in these patients. With multivariable Cox regression analysis, four parameters emerged as independently associated with the combined end-point: peak aortic jet velocity (p=0.04), LV longitudinal myocardial deformation (p=0.03), valvulo-arterial impedance (p=0.001) and indexed left atrial area (p=0.007) (table 2). Using receiver–operator characteristic curve analysis (figure 2), a peak aortic jet velocity ≥4.4 m/s, a LV longitudinal myocardial deformation ≤15.9%, a valvular-arterial impedance ≥4.9 mm Hg/ml per m2 and an indexed left atrial area ≥12.2 cm2/m2 were identified as the best cut-off values to be associated with events (figure 3). When these were entered as categorical parameters in the same multivariable model as in table 2, all remained significant.
Demographic, clinical and echocardiographic characteristics*
Univariable and multivariable analysis of event-free survival
Receiver−operator characteristic curves analysis for the prediction of events. Cut-off values were indicated using a square for indexed left atrial area (12.2 cm2/m2, area under the curve [AUC]=0.62), a triangle for longitudinal strain (15.9%, AUC=0.70), a circle for valvulo-arterial impedance (4.9 mm Hg/ml/m2, AUC=0.74) and a lozenge for peak aortic velocity (4.4 m/s, AUC=0.83).
Kaplan–Meier event-free survival curves according to categorical variables that were selected in the Cox proportional-hazards regression analysis. Peak aortic velocity (A), valvulo-arterial impedance (B), longitudinal strain (C), indexed left atrial area (D). The mean±SD survival rates at two and 4 years are indicated. Ao denotes aortic, V-Ai valvulo-arterial impedance, Long. longitudinal and LA left atrial.
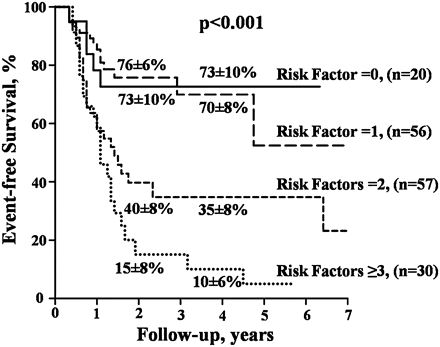
In the population, 57 patients (34%) cumulated two risk markers and 30 (18%) had three or four predictive markers. The RR of events rose with the increased number of risk markers (figure 4). At 2 years, the probability of remaining free of cardiac events was 76±6% in the patients with no risk marker, 73±10% in the patients with one risk variable, 40±8% in the patients with two risk markers and 15±8% in the patients with three or four risk markers (p<0.001).
Kaplan–Meier event-free survival curves according to the cumulative number of risk markers (indexed left atrial area, valvulo-arterial impedance, maximum aortic jet velocity, longitudinal strain). The mean±SD survival rates at 2 and 4 years are indicated.
During the first year following inclusion, 49 patients had an event (32% of the patients with follow-up >1 year). In multivariable logistic regression, valvulo-arterial impedance, LV longitudinal strain and indexed left atrial surface came out as independent determinants of 1-year event-free survival (table 3).
Univariable and multivariable analysis of 1-year event-free survival
To note in the six patients who died, the valvulo-arterial impedance was 5.8±0.9 mm Hg/ml per m2 and the aortic valve area 0.39±0.02 cm2/m2. Death occurred at a mean follow-up of 10±5 months.
On the other hands, in patients with a negative exercise test, peak aortic jet velocity (p=0.03), LV longitudinal myocardial deformation (p=0.012), valvulo-arterial impedance (p=0.035) and indexed left atrial area (p=0.003) remained associated in the multivariable model with the outcomes.
Reproducibility of measurements
The reproducibility of measurements was tested by random selection of 15 patients enrolled in Rennes. There were good interobserver agreements for LV volumes (r=0.87), LV ejection fraction (r=0.85), aortic pressure gradients (r=0.91), stroke volume (r=0.92) and aortic valve area (r=0.88). Low coefficients of variation were found between observers with mean relative differences of 6.1% for all the parameters (range 4.6–12.4%). All measurements concerning myocardial deformation were performed in Liège. The inter- (r=0.89) and intraobserver (r=0.86) regression coefficients for LV longitudinal myocardial deformation have been reported as good by our group.9
Discussion
In this prospective study of 163 patients with asymptomatic moderate to severe AS, we identified four Doppler-echocardiographic parameters that were strongly associated with the occurrence of cardiac events: aortic jet velocity, a measure of stenosis severity; valvulo-arterial impedance, an estimate of global LV afterload; left atrial area index, a marker of LV diastolic dysfunction; and LV longitudinal deformation, an indicator of subclinical LV systolic dysfunction. In contrast, no clinical variables were independently associated with outcome. At 2 years, only 43% of patients had an uneventful clinical course. The likelihood of remaining free of cardiac events decreased substantially according to the number of risk markers. These data emphasise that the prognosis of patients with asymptomatic AS depends not only on stenosis severity but also on the level of LV load and its consequences on LV function.
The management of asymptomatic patients with severe AS is controversial. The immediate risks of AVR are weighed against the later risk of events without surgical intervention. Physicians may feel uncomfortable about delaying surgery until symptoms occur. Indeed, many patients do not report their symptoms promptly, some patients deny symptoms and the risk of death is significant if the patients need to wait several months for surgery.11
Previous studies have shown that the outcome of patients with asymptomatic AS could be dependent on several clinical, biological and echocardiographic variables: age, functional status, chronic renal failure, C-Reactive protein level, severity of stenosis and its rate of progression and the extent of valve calcification.12–14 The occurrence of symptoms during an exercise test can identify patients who will develop symptoms during following year, but elderly and/or inactive patients are not always able to exercise.15 16 In the present study, although an abnormal response to exercise was associated with the outcome in univariable analysis, it did not emerge as an independent predictor in the multivariable model. This could be related to the subjectivity of symptoms during the test and its low predictive value in elderly subjects.15
In this study, age, mean pressure gradient, LV mass, LV volumes and ejection fraction were similar in patients who developed an event and in those who remained asymptomatic over the study period. Mean age of our patients was 70 years and the majority of them had moderate or severe valve calcification. Our results confirm that the severity of AS is a strong predictor of outcome. Although mean pressure gradient was similar in the two groups, indexed valve area was significantly lower in patients who had cardiac events. Aortic jet velocity was selected on multivariable analysis as a stronger predictor of prognosis and particularly of mid-term outcome. Jet velocity is recorded directly on Doppler examination, is reproducible, and does not require calculations.13 These data are in line with recent publications.17 Rosenhek et al have showed that patients with very severe AS have a poor prognosis with a high event rate and a risk of rapid functional deterioration. In our study, 15 of 20 patients with an aortic jet velocity >5 m/s developed cardiac events during follow-up (15/20 vs 59/84, p=0.004).
AS cannot be viewed as an isolated disease of the valve.18 The prevalence of atherosclerosis and hypertension is high in these patients. Both conditions can accelerate arterial stiffness.19 The increase in LV afterload does not only result from outflow obstruction but also from reduced systemic arterial compliance. A recent approach to assess global afterload incorporates the degree of valve stenosis and the systemic arterial compliance by calculating valvulo-arterial impedance.10 Mean pressure gradient and stroke volume index are obtained by Doppler echocardiography. Systolic arterial pressure is included in the numerator of the formula and should thus be recorded systematically at the time of echocardiographic examination. In retrospective studies, a valvulo-arterial impedance >5 mm Hg/ml/m2 has been found to be independently associated with a 4-fold increase in risk of LV dysfunction and a value of >5.5 mm Hg/ml/m2 was associated with a 2.5-fold increase in risk of mortality in patients with symptomatic or asymptomatic AS.20 Our prospective study confirms that patients with increased valvulo-arterial impedance had a higher risk of developing events. Increased global afterload was notably associated with early cardiac events during the first year of follow-up.
Prolonged high LV global afterload can exceed the limit of LV compensatory mechanisms and lead to an intrinsic impairment of myocardial function. When cellular adaptation is exhausted, LV filling pressure increases, producing increased left atrial wall tension and myocyte stretch inducing myolysis, fibrosis, apoptosis and, in turn, atrial enlargement.21 Left atrial size increases with worsening diastolic dysfunction and reflects the magnitude and the chronicity of the increased LV filling pressure.22 In the present study, left atrial area index was found to be a powerful prognostic marker. About one-half (46%) of our patients had a significant enlargement of the left atrium. In this subgroup of patients, the 2-year probability of events was 77%. After surgery, Rossi et al showed that left atrial size can predict postoperative symptomatic improvement.23
In the pressure overloaded myocardium, continuous turnover of the extracellular matrix occurs during the progression from compensatory hypertrophy to heart failure. A longstanding increase in global LV afterload results in LV concentric hypertrophy.24 For a similar extent of intrinsic myocardial shortening, the LV ejection fraction tends to increase in relation to the extent of LV concentric remodelling.24 Although controversial, weight of opinion is considered in favour of surgery in asymptomatic patients with severe AS who present with an ejection fraction below 50%.4 5 However, LV ejection fraction remains frequently normal.20 Indeed, the ejection fraction was identical in the two groups of our population. Longitudinal function is governed by the subendocardial myocardial fibres which are more likely to be affected by microvascular ischemia.25 In hypertrophic myocardium, myocardial blood flow is maldistributed, and oxygen supply to the endocardium is limited. In addition, subendocardial function of the left ventricle may be depressed in patients whose conduct arteries are stiffer and less compliant than normal.19 The LV longitudinal function can be reliably quantitated by the measurement of myocardial deformation from the speckle tracking analysis.16 25 In asymptomatic patients with AS, impaired subendocardial function has been shown to be associated with impaired exercise tolerance and changes in symptomatic status during short-term follow-up.25–28 Our results extend these preliminary data. Reduced longitudinal contraction identified a subset of patients at higher risk of developing cardiac events. Patients with LV longitudinal strain ≤15.9% had an excess risk of death, symptoms or surgery that was more than twice that of patients with preserved longitudinal function.
Our results strengthen the need for a comprehensive evaluation of asymptomatic patients with AS that goes beyond the standard measures of mean pressure gradient and aortic valve area. They highlight the combined role of both outflow obstruction and the peripheral circulation and their consequences on LV systolic and diastolic function. Patients with no or only one risk marker could be followed until symptoms develop. Patients who combine two risk markers should be carefully followed as 60% event rate at 2 years can be expected. In the presence of three or four risk markers, early AVR should be considered as such patients represent a very high-risk group.
Study limitations
In the present study, we did not assess the presence and extent of coronary atherosclerosis in patients who were not submitted to surgery. To note, 39 patients underwent coronary angiography during follow-up. Multivessel disease was observed in 22, single-vessel lesions in four and non-significant coronary stenosis (<50%) in 13. As the present study was performed before the European recommendations of 2007, the results of the exercise test likely had no or minimal impact on patient management. The decision to perform surgery was made by individual cardiologists in charge of the patients. AVR could be considered as a soft event. However, in the present study, surgery was mainly dictated by the onset of symptoms, which are currently a class I indication for intervention. Moreover, serial echocardiographic examinations were available in a limited number of patients. Therefore, the prognostic importance of the rate of progression could not be evaluated. Finally, all measurements made in the present study may lengthen analysis. However, it should be highlighted that their analysis in the setting of AS are currently recommended. The analysis of LV myocardial deformation and the calculation the valvulo-arterial impedance take > or <2 min. Finally, all these measurements are exposed to source of errors and required learning curves.
Conclusions
In patients with asymptomatic moderate to severe AS, measurements that integrate the ventricular, vascular and valvular components of the disease improve risk stratification and may help to identify patients who could benefit from an early elective aortic valve surgery.
References
Footnotes
Competing interests None.
Patient consent Obtained.
Ethics approval This study was conducted with the approval of the Liège CHU Sart Tilman and CHU de Ponchaillou, Rennes, FRANCE.
Provenance and peer review Not commissioned; externally peer reviewed.