Video capsule colonoscopy in routine clinical practice
Introduction
Since introduced into routine clinical practice in the late 1960s, conventional or optical colonoscopy has become the gold standard for the investigation of colon diseases. It has proven invaluable for colorectal cancer (CRC) screening and inflammatory bowel disease (IBD) diagnosis and surveillance amongst other clinical applications. However, conventional colonoscopy (CC) remains an invasive, labour- and resource-intensive procedure which is associated with certain risks and adverse events, including perforation, haemorrhage and the risks associated with sedation or even anaesthesia if required (1,2). Furthermore, it is an uncomfortable, undignified experience for many patients with several points of contention, from the procedure itself to the laxative bowel preparation required beforehand (1). This has been shown to discourage and limit patient uptake of colonoscopic investigation and treatment. In an audit of the British national bowel cancer screening programme, up to 10% of patients experienced significant discomfort during colonoscopy, with about 35% of patients reporting some discomfort from the procedure (3,4). A systematic review by Doyle et al. has shown that patient experience is positively associated with clinical effectiveness and patient safety (5). The current uptake of CRC screening remains lower than ideal (6) and patient comfort and acceptance should therefore not be overlooked.
Although computed tomography (CT) colonography has been used as an alternative in patients unable or unwilling to undergo CC, it is less accurate (7-9) and does not offer direct visualisation of the bowel mucosa. Capsule endoscopy (CE) has been adopted as the prime modality for non-invasive small bowel imaging (10). Likewise, colon CE (CCE) has recently been developed to fulfil a similar role, offering the advantages of direct mucosal visualisation without the need for sedation or gas insufflation (11). The European Society of Gastrointestinal Endoscopy (ESGE) has determined that CCE is generally feasible, safe and accurate for use in patients with incomplete colonoscopy, and although it acknowledges the current lack of sufficient evidence, it allows that CCE may have a role in IBD surveillance (12). The current evidence for the role of CCE as an adjunct or alternative to CC remains equivocal (2,13-15). Therefore, in this observational cohort study, we report our clinical experience of using CCE to investigate patients with suspected colon pathology at a tertiary referral centre in Sweden.
Methods
From November 2007 to November 2015, consecutive patients with incomplete CC or who had refused further investigation with colonoscopy were recruited from a tertiary care centre in Malmo, Sweden. Full informed consent was obtained from these patients who underwent CCE using the PillCam® COLON 1 (CCE-1; Given® Imaging Ltd., Yokneam, Israel) and 2 (CCE-2; Covidien, Minneapolis, USA). As per local protocol patients undertook a low residue diet for 3 days prior to CCE with a clear liquid diet on the day before. The evening before the examination, patients ingested 3 L of polyethylene glycol (PEG) solution, followed by an additional 1 L on the morning of the procedure. The patient then swallowed the capsule. When the capsule was seen in the small bowel using the real-time viewer, the patients were given a booster dose of 30 mL of sodium phosphate (NaP) solution with 1 L of water. Further progression of the capsule was followed throughout the examination using the Rapid Access real-time viewing system. If the capsule was not excreted within 3 h of the first booster dose of NaP, a second booster dose of 15 mL NaP in 0.5 L water was provided. After another 2 h, if the capsule was still not expelled, a further 10 mg bisacodyl suppository was administered. The bowel preparation schedule is detailed in Table 1.

Full table
Data collected were: patient demographics (age, gender), indication for CCE, CCE findings, bowel cleansing, colon transit time (CTT) and completeness of colon examination. A complete CCE examination was defined as natural excretion of the capsule, as per Spada et al. (11). Results are reported as median (range) for continuous data and percentages for discrete data.
Results
Over the study duration, a total of 77 consecutive patients (57 females, 20 males) with suspected colon pathology were included. The median age of the group was 56 years (range, 15–89 years). Thirty-nine patients had had a previously incomplete CC and 29 patients had declined CC. The main clinical indications were gastrointestinal (GI) bleeding (n=28; 36%) and suspected IBD or follow-up in patients with known IBD (n=23; 30%); other indications included abdominal pain/diverticular disease (n=18; 23%), CRC screening (n=3), follow up of abnormal radiology (n=3) and follow up after polypectomy (n=2). Patient characteristics in our group are summarised in Table 2.
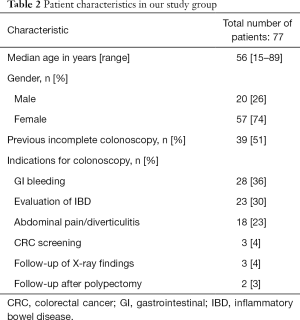
Full table
Colon examination was complete in 58/77 (75%) patients with a median CTT of 257 min (range, 3–895 min). CCE was conducted using CCE-1 in 42 patients and CCE-2 in 35. Completion rates were similar: 32/42 (76%) using CCE-1 and 26/35 (74%) using CCE-2. The capsule did not reach the colon in 3 patients due to stomach retention, retention at a small-bowel stricture and slow small bowel transplantation (SBT). In the remaining 16 incomplete examinations, the capsule reached the rectum (n=4), sigmoid (n=6), descending colon (n=5) and transverse colon (n=1). Good or excellent bowel preparation was achieved in 58 (75%) patients. Patient outcomes are detailed in Table 3.
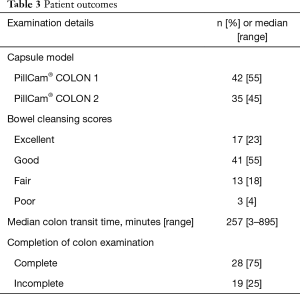
Full table
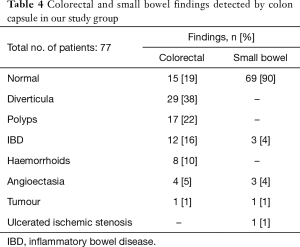
The most frequent findings were colonic diverticula (n=29; 38%), polyps (n=17; 22%; size 3–20 mm), active IBD (n=12; 16%), haemorrhoids (n=8; 10%), colonic angioectasia (n=4; 5%) and advanced cancer (n=1; 1%), Figure 1. Fifteen (19%) patients had no colon pathology on CCE. Furthermore, small-bowel findings were recorded in 8 (10%) patients, including stricture, angioectasia, tumours and lesions consistent with Crohn’s disease (Figure 2). The findings in our group are summarised in Table 4. All patients in our cohort tolerated the bowel preparation and the CCE procedure well. Two patients with significant pathology (ulcerated small-bowel stricture and CRC) experienced temporary capsule retention during the examination with spontaneous resolution. In both patients, the CCE was excreted within 7 days following the examination, as verified by plain abdominal X-ray.
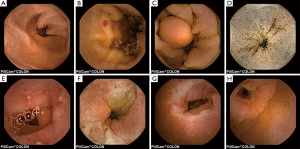
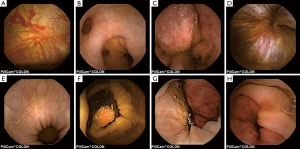
Full table
Discussion
CCE is currently provided by a single model of capsule, the PillCam® Colon. The first-generation CCE-1 was introduced in 2006; a main difference between the CCE and conventional small-bowel CE was the inclusion of 2 cameras enabling image capture from both ends of the capsule. The CCE-2 introduced a wider field of view of 172° for each camera, therefore achieving nearly 360° views. The CCE-2 also has adaptive frame rate to conserve battery life, taking images from 4 images per second when the capsule is immobile to 35 images per second when movement is detected (16). Currently, the ESGE recommends the use of CCE in average-risk patients where CC is contraindicated, technically impossible or strongly opposed by the patient (12). In our cohort of patients, CCE presented a viable alternative to CC; however this observational study highlights some of the limitations still inherent to the procedure.
Despite the widespread use of CC, 4–20% of such examinations are incomplete, usually due to anatomical reasons (14); furthermore the discomfort involved with such procedures could deter patients from repeat attempts where necessary. The alternative mode of investigation, CT colonography, has lower accuracy for flat lesions (14,17-19) which have been associated with increased malignant potential, whereas CCE offers direct mucosal visualisation without radiation. However, in studies using CCE-1, the sensitivity and specificity for polyps ≥6 mm were 64% and 84% respectively. Using CCE-2, sensitivity and specificity improved to 83% and 89%, with polyps as small as 6 mm detected (2).
In a meta-analysis by Spada et al., the overall completion rate of examinations carried out using CCE-1 was 86.7%, improving to 90.5% using CCE-2 (20). This falls short of standard cecal intubation rates for CC. In fact, in earlier studies using CCE, the completion rate was as low as 70% (20). Interestingly, our cohort had a low completion rate of only 75%. There was no significant difference in completion rates using CCE-1 and CCE-2; however it must be noted that all CCE-2 examinations in our group were performed using a lower dose of NaP (30+15 mL compared to the 45+30 mL used in the CCE-1 examinations). This change was made by our centre to reduce the risk of adverse events associated with NaP (12). Although examination was incomplete in 25% of the patients in our cohort, a key difference between CCE and CC is that the capsule travels from proximally to distally; in 15/16 patients with incomplete CCE, the proximal colon was examined. Studies have shown that in patients with incomplete colonoscopy, the incidence of proximal CRC is increased up to 2-fold (21); furthermore there is an increased risk of missing a concurrent proximal CRC (22-24).
Nevertheless, inadequate bowel preparation and visualisation remains the biggest cause of incomplete CCE (14). Although meta-analyses have shown that laxative bowel preparation is crucial, they also quote low median rates of adequate cleansing: 78% in CCE-1 and 81% in CCE-2 (20). This is similar to the rate of adequate visualisation obtained in our cohort. At present, the ESGE suggests the use of a total of 4 L PEG, given in split doses the day before and on the day of CCE examination, as well as post-capsule ingestion NaP boosters in patients with no contraindications (12). However, the evidence to support this practice is lacking (12). Furthermore, the intensity of the bowel preparation is a strong deterrent for the wider adoption of CCE, negating the non-invasiveness of the capsule itself (15,25).
In our cohort, there were only two cases of transient retention of the CCE. One case occurred in a patient with a small-bowel stricture, highlighting perhaps the importance of screening patients for risk of retention as one would for small-bowel CE. This will become a more pertinent issue with the wider uptake of CCE in future. There is currently limited data on the use of CCE in IBD as the majority of studies have dealt with the use of CCE in CRC screening. Overall, the sensitivity and specificity of CCE for ulcerative colitis was 89% and 75% compared to CC (14). However, it may have a role in monitoring in relatively stable patients, due to the potential of avoiding colonoscopies at certain intervals.
Limitations include the retrospective nature of this observational study, which was conducted at only a single centre. However, ours is a tertiary care centre serving a wide population in the southern part of Sweden which has about 1.3 million inhabitants, therefore providing a realistic view on the use of CCE in day-to-day clinical practice. The low numbers of patients referred for CCE roughly reflect the limited dissemination of this method amongst practising physicians in the area. Our study shows that CCE is a relatively well-tolerated alternative to CC, but requires technological improvement and optimisation of clinical practice to bring it in line with the current reference standard. A cost-benefit analysis has estimated that use of CCE for population screening would be beneficial if uptake of screening could be increased (26). Although further technical development may be needed to examine the whole colon in large numbers of patients, CCE may complement or even replace CC for certain clinical indications.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Yung has received grant from Dr. Falk Pharma/Core and travel support from Dr. Falk Pharma; Dr. Koulaouzidis has received European Society of Gastrointestinal Endoscopy (ESGE) Given Imaging grant, material support for research from SynMed UK, honoraria from and member of advisory board for Dr. Falk Pharma UK and travel support from Almirall & Dr. Falk Pharma; Dr. Toth has received lecture fees from Given Imaging/Medtronic; Dr. Wurm Johansson has received lecture fees from Medtronic. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, in compliance with good clinical practice and local regulations and the study was approved by the Ethical Committee of Lund University, Sweden (approval number: 2016/412). All patients gave written informed consent prior to the examinations.
References
- Fisher DA, Maple JT, Ben-Menachem T, et al. Complications of colonoscopy. Gastrointest Endosc 2011;74:745-52. [Crossref] [PubMed]
- Carter D, Eliakim R. PillCam colon capsule endoscopy (PCCE) in colonic diseases. Ann Transl Med 2016;4:307. [Crossref] [PubMed]
- Lee TJ, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut 2012;61:1050-7. [Crossref] [PubMed]
- Logan RF, Patnick J, Nickerson C, et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439-46. [Crossref] [PubMed]
- Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013;3:e001570. [Crossref] [PubMed]
- Bujanda L, Sarasqueta C, Zubiaurre L, et al. Low adherence to colonoscopy in the screening of first-degree relatives of patients with colorectal cancer. Gut 2007;56:1714-8. [Crossref] [PubMed]
- IJspeert JE, Tutein Nolthenius CJ, Kuipers EJ, et al. CT-Colonography vs. Colonoscopy for Detection of High-Risk Sessile Serrated Polyps. Am J Gastroenterol 2016;111:516-22. [Crossref] [PubMed]
- Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 2009;58:241-8. [Crossref] [PubMed]
- Rosman AS, Korsten MA. Meta-analysis Comparing CT Colonography, Air Contrast Barium Enema, and Colonoscopy. Am J Med 2007;120:203-10. [Crossref] [PubMed]
- Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015;47:352-76. [Crossref] [PubMed]
- Spada C, De Vincentis F, Cesaro P, et al. Accuracy and safety of second-generation PillCam COLON capsule for colorectal polyp detection. Therap Adv Gastroenterol 2012;5:173-8. [Crossref] [PubMed]
- Spada C, Hassan C, Galmiche J, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2012;44:527-36. [Crossref] [PubMed]
- Friedel D, Modayil R, Stavropoulos S. Colon Capsule Endoscopy: Review and Perspectives. Gastroenterol Res Pract 2016;2016:9643162. [Crossref] [PubMed]
- Triantafyllou K, Beintaris I, Dimitriadis GD, et al. Is there a role for colon capsule endoscopy beyond colorectal cancer screening? A literature review. World J Gastroenterol 2014;20:13006-14. [Crossref] [PubMed]
- Yung DE, Rondonotti E, Koulaouzidis A. Capsule colonoscopy — a concise clinical overview of current status. Ann Transl Med 2016;4:398. [Crossref] [PubMed]
- Spada C, Hassan C, Costamagna G. Colon Capsule Endoscopy. Gastrointest Endosc Clin N Am 2015;25:387-401. [Crossref] [PubMed]
- Figueiredo PN, Figueiredo IN, Prasath S, et al. Automatic Polyp Detection in Pillcam Colon 2 Capsule Images and Videos: Preliminary Feasibility Report. Diagn Ther Endosc 2011;2011:182435. [Crossref] [PubMed]
- Rondonotti E, Borghi C, Mandelli G, et al. Accuracy of capsule colonoscopy and computed tomographic colonography in individuals with positive results from the fecal occult blood test. Clin Gastroenterol Hepatol 2014;12:1303-10. [Crossref] [PubMed]
- Spada C, Hassan C, Barbaro B, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut 2015;64:272-81. [Crossref] [PubMed]
- Spada C, Pasha SF, Gross SA, et al. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1533-43. [Crossref] [PubMed]
- Brenner H, Chang-Claude J, Jansen L, et al. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: A population-based case-control study. Ann Intern Med 2012;157:225-32. [Crossref] [PubMed]
- Villa NA, Pannala R, Pasha SF, et al. Alternatives to Incomplete Colonoscopy. Curr Gastroenterol Rep 2015;17:43. [Crossref] [PubMed]
- le Clercq CM, Winkens B, Bakker CM, et al. Metachronous colorectal cancers result from missed lesions and non-compliance with surveillance. Gastrointest Endosc 2015;82:325-333.e2. [Crossref] [PubMed]
- Stoffel EM, Erichsen R, Froslev T, et al. Clinical and Molecular Characteristics of Post-Colonoscopy Colorectal Cancer: A Population-based study. Gastroenterology 2016;151:870-8. [Crossref] [PubMed]
- Belsey J, Epstein O, Heresbach D. Systematic review: Oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007;25:373-84. [Crossref] [PubMed]
- Palimaka S, Blackhouse G, Goeree R. Colon Capsule Endoscopy for the Detection of Colorectal Polyps: An Economic Analysis. Ont Health Technol Assess Ser 2015;15:1-43. [PubMed]


