A 29-year-old woman receiving immunosuppressants for bilateral lung transplant performed 14 years earlier because of a surfactant protein C deficiency presented with a 1-month history of worsening watery diarrhea, nausea, vomiting, abdominal cramping, and subjective fevers. She said she had no hematochezia, hematemesis, suspicious food intake, recent contact with sick people, or family history of inflammatory bowel disease.
Her medications included tacrolimus, mycophenolate, and low-dose prednisone with no recent dosage adjustments. Her most recent tacrolimus level, measured 3 months earlier, was 8.8 ng/mL (reference range 10–20 ng/mL), and her average absolute lymphocyte count was 4.78 × 109/L (reference range 1.18–3.74 × 109/L). The physical examination was unremarkable except for mild generalized abdominal tenderness and signs of dehydration.
INVESTIGATING THE CAUSE
The patient was admitted for intravenous fluid administration and diarrhea workup. The differential diagnosis included a broad array of typical and opportunistic gastrointestinal infections, especially those with a higher incidence in immunosuppressed hosts, including parasites (eg, Giardia, Cryptosporidium), bacteria (eg, Clostridioides difficile, Campylobacter), and viruses (eg, cytomegalovirus). Noninfectious causes including medication toxicity, immunologic reactions, inflammatory bowel disease, and malignancy also were considered.
Results of the complete blood cell count were normal. Her aminotransferase levels were mildly elevated, with an alanine aminotranserase of 60 U/L (reference range 6–55 U/L) and aspartate aminotransferase of 41 U/L (reference range 5–34 U/L); alkaline phosphatase and total bilirubin levels were normal. Her tacrolimus level was 16.2 ng/mL. A quantitative cytomegalovirus DNA test was negative.
Stool tests for fecal leukocytes, ova and parasites, C difficile toxin (using a polymerase chain reaction test), Shiga toxins, Giardia antigen, and pancreatic fecal elastase were normal. The fecal calprotectin level was 189 μg/g (reference range 0–120 μg/g).
Further workup
Abdominal computed tomography with contrast enhancement showed no acute intra-abdominal abnormalities.
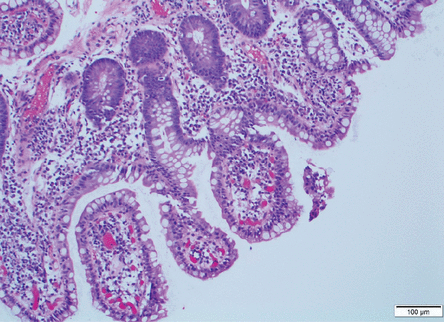
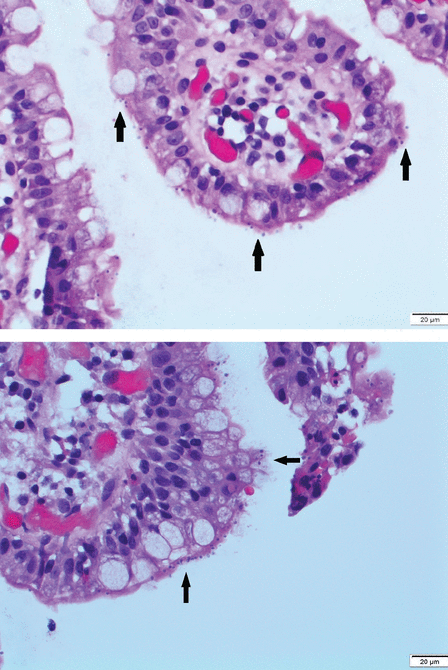
The patient underwent upper endoscopy and colonoscopy, with normal findings. Multiple biopsies were taken, including some of the terminal ileum. Results showed nonspecific villous blunting (Figure 1) and many mucosal apical basophilic circular-shaped organisms with epicellular invasion and marked increased lymphoplasmacytic infiltrate in the lamina propria (Figure 2). All of these are consistent with cryptosporidiosis.
Hematoxylin and eosin stain of terminal ileum biopsy (scale bar 100 μm) revealed marked increased lymphoplasmacytic infiltrate in the lamina propria, with villous blunting.
Hematoxylin and eosin stain of terminal ileum biopsy (scale bar 20 μm) revealed many mucosal, apical, basophilic, circular-shaped organisms, with epicellular invasion (black arrows).
Stool sample immunofluorescent assays for Cryptosporidium oocyst antigens later confirmed the diagnosis of cryptosporidiosis.
CRYPTOSPORIDIOSIS
Cryptosporidium parvum and Cryptosporidium hominis are unicellular parasites that usually cause self-limited diarrhea in immunocompetent hosts; however, they can cause life-threatening diarrhea in immunocompromised patients, especially those with impaired cell-mediated immunity or interferon-gamma production.1
The pathophysiology of diarrhea remains unclear. Theories suggest a combination of malabsorption and secretory diarrhea secondary to mucosal attachment, villous architecture distortion, epicellular invasion, inflammatory response, and cellular apoptosis.1–2 Cryptosporidia can also involve the biliary system by spreading through the intestinal lumen.
Direct immunofluorescence and polymerase chain reaction testing are the most sensitive and specific diagnostic tests for cryptosporidiosis.3 Although routine stool examination for ova and parasites remains the simplest and most widely available test, its sensitivity is limited by low levels of oocyst shedding and variable microscopic expertise.
In general, microscopy of tissue biopsies from the gastrointestinal tract is limited by the patchy distribution of infection; however, it was the key to the diagnosis in our patient.
Therapy in immunocompromised patients, other than those with human immunodeficiency virus infection, involves reducing the dose of immunosuppressive therapy and initiating dual antibiotics.4
We treated our patient with 14 days of nitazoxanide 500 mg twice daily and azithromycin 500 mg daily and reduced her mycophenolate dose, and her symptoms resolved.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.