ABSTRACT
Any survivor among the millions of patients admitted to the intensive care unit (ICU) for critical illness each year is susceptible to persistent health problems that continue after discharge and may lead to post-intensive care syndrome (PICS), defined as new or worsening dysfunction from physical impairment, cognitive impairment, or emotional impairment, or a combination. Considering the increased rates of ICU survival and the growing elderly population more likely to utilize ICU resources, critical care practitioners have broadened their focus on outcomes and care of ICU survivors to include the acute post-ICU survival period as well as months and even years after ICU discharge. This review focuses on the neuropsychiatric aspects of PICS in ICU survivors including diagnostic, screening, and treatment recommendations. It also highlights the value of post-ICU clinics and the unique role of the consultation psychiatrist in the care of this patient population.
From 50% to 70% of the millions of patients admitted to the ICU each year will experience PICS, defined as new or worsening dysfunction in one or more of the following domains: physical impairment, cognitive impairment, and emotional impairment.
Critical care practitioners have broadened their focus of post-ICU care to include more than just the typical acute post-ICU care of 30 days after discharge.
To help navigate post-ICU care for survivors, post-ICU clinics have been developed where ICU survivors can receive outpatient follow-up care from a multidisciplinary team of providers to address their targeted needs.
Millions of patients are admitted to the intensive care unit (ICU) annually in the United States.1 The most frequent diagnoses associated with ICU admissions include the following:
Respiratory failure including acute respiratory distress syndrome (ARDS)
Acute myocardial infarction
Cerebral infarction
Percutaneous cardiovascular procedures
Severe sepsis or septic shock.1
Considering the increased rates of ICU survival (currently 71% to 90%)1 and the growing elderly population (20% of the global population will be over age 65 by 2050),2 more people are likely to utilize ICU resources.
Any survivor of a critical illness and ICU stay is susceptible to health problems that continue to persist after discharge and may lead to post-intensive care syndrome (PICS). PICS was designated as a syndrome by the Society of Critical Care Medicine in 2010,3 occurs in 50% to 70% of ICU survivors,4 and is defined as new or worsening dysfunction in one or more of the following domains: physical impairment, cognitive impairment, and emotional impairment. We will explore each of these domains through a psychiatric lens.
As a result, critical care practitioners have broadened their focus on outcomes and care of ICU survivors to include the acute post-ICU survival period (30 days after ICU discharge) as well as the months and even years after ICU discharge. Post-ICU recovery care is even more necessary during the COVID-19 pandemic as early studies noted ICU admission rates of 32% of all COVID-19 patients,5,6 increasing the number of ICU survivors in need of care.
This review focuses on neuropsychiatric aspects of care of ICU survivors, particularly regarding symptoms associated with PICS including neuropsychiatric diagnostic, screening, and treatment recommendations, as well as the value of post-ICU recovery clinics.
POST-INTENSIVE CARE SYNDROME
Physical impairment
ICU-acquired weakness can be categorized as critical illness polyneuropathy, critical illness myopathy, and critical illness neuropathy and myopathy. It may affect up to half of ICU survivors admitted for 1 week or more.1 Specifically, about two-thirds of mechanically ventilated patients, 60% of patients with adult respiratory distress syndrome, and half of patients with sepsis will experience some degree of ICU-acquired weakness.7,8
Several aspects of critical illness contribute to ICU-acquired weakness, from the cellular level (mitochondrial dysfunction, release of inflammatory cytokines) to systemic concerns such as inactivity and malnutrition.2 Patients with ICU-acquired weakness who also have comorbid cognitive or emotional dysfunction may be less able to participate in physical rehabilitation and other therapies to improve weakness, thus placing them at further risk of prolonged physical weakness and highlighting the importance of targeted prevention and intervention for overall mental and physical recovery.
Other important aspects of physical morbidity are exercise limitation, fatigue, joint immobility, impairment of activities of daily living, shortness of breath, hair loss, voice changes, dysphagia, and sexual dysfunction.9–11 All of these impairments may affect quality of life and can subsequently interfere with the mental health of ICU survivors.
Cognitive impairment
ICU survivors are at risk of acute and chronic cognitive dysfunction.12–18 From 20% to 40% of ICU survivors experience persistent cognitive impairment, an undeniable major complication of critical illness that most commonly affects cognitive areas of executive function, attention, and memory.12 Cognitive dysfunction in ICU survivors has been associated with decreased quality of life, even in patients who recover physically.19 Some patients with persistent cognitive impairment are no longer able to work. Studies have shown that 30% to 38% of patients were able to return to work 3 months after ICU discharge.13–15 At 12 months post-ICU, 42% to 58% of patients were able to return to work.13,16–18 Depending on the severity of the cognitive impairment, patients’ family members are sometimes obligated to forfeit their social and occupational roles and adopt a new role of caregiver; this can be a significant financial burden for patients and families and also has societal impact considering substantial productivity loss.4
Delirium. Delirium is a well-known cognitive complication of ICU admission, affecting up to 75% of ICU patients with an increased incidence in mechanically ventilated patients.20 Pandharipande et al20 noted a longer duration of delirium to be associated with worse global cognition and executive function at 3 and 12 months and with worsening depressive symptoms and quality of life 1 year after ICU discharge. Pathophysiologic causes of delirium include acute inflammatory responses, metabolic derangements—particularly hyperglycemia and hormonal disturbances, and toxic or medication-induced delirium from exposure to benzodiazepines, opiates, sedatives, hypnotics, steroids, and anticholinergic medications.12
Delirium is not always associated with persistent cognitive impairment as many patients recover cognitive function with treatment of their underlying medical conditions. However, Gunther et al linked delirium duration to brain changes in patients admitted to the ICU with respiratory failure or shock.21 Longer delirium duration was independently associated with smaller overall brain volumes on magnetic resonance imaging (MRI) as well as smaller superior frontal lobe volumes at hospital discharge and 3-month follow-up. Significantly smaller hippocampal volumes were noted at time of discharge in patients with increased delirium duration; these differences were statistically significant, but there was not a statistically significant difference at 3-month follow-up. Serial MRI studies have shown decreased thalamic and cerebellar volumes at 3-month follow-up in patients with longer periods of hospital delirium that were associated with worse executive functioning and visual attention impairment at 12 months post-ICU.21
Delirium in COVID-19 patients. Delirium has been identified in 10% to 30% of COVID-19 hospitalized patients.22,23 The incidence of delirium that can present even in the absence of respiratory symptoms in COVID-19 ICU patients is not precisely known, but estimates range from 50% to 80%.24,25 Furthermore, management of delirium associated with COVID-19 involves a step-based pharmacologic intervention protocol established by Massachusetts General Hospital with a graduated progression from melatonin, to alpha-2 agonists, to low-potency antipsychotics, to valproic acid and dopamine agonists.26
Delirium risk factors. There are several nonmodifiable pre-ICU risk factors for delirium including older age, lower level of education, pre-existing cognitive impairment, acute severity of illness, and presence of the apolipoprotein E epsilon 4 allele or major genetic risk factor for Alzheimer disease (even in the absence of major neurocognitive disorder).12 Thus, practitioners need to identify and implement prevention strategies for potentially modifiable risk factors for delirium including sleep hygiene, frequent reorientation, assurance that sensory augmentation devices are provided (eyeglasses, hearing aids), avoidance of deliriogenic medications (narcotics, hypnotics, anticholinergics), metabolic and hemodynamic stability, and appropriate sedation weaning. Delirium prevention is reviewed later in this article including the use of the Society of Critical Care Medicine ICU Liberation Bundle (A–F).27,28
Emotional impairment
Up to one-third of ICU survivors may experience a range of psychiatric dysfunctions after discharge.29 Patients with emotional impairment related to PICS are more likely to experience decreased quality of life.29 For the purpose of this article, emotional impairment encompasses psychiatric, psychological, and mental health symptoms.
Depression. Post-ICU depression affects about 30% of ICU survivors and is associated with increased medical admissions and emergency department visits.30 Of note, patients with post-ICU depression more often report somatic symptoms (fatigue, decreased physical energy, psychomotor slowing) rather than cognitive-affective symptoms. These symptoms can be difficult to differentiate from physical symptoms of critical illness. Somatic symptoms of depression are less likely to respond to antidepressant medications and may require more comprehensive treatment strategies.30 The BRAIN-ICU study reported that severe depressive symptoms in the early post-ICU period (first 3 months) were likely to persist as 33% of the study population experienced at least mild depressive symptoms at 3-month follow-up that continued at 12-month follow-up.31
Anxiety. The prevalence of anxiety in ICU survivors is estimated to be about 70%.4 Patients with post-ICU anxiety often have comorbid post-ICU depression or post-traumatic stress disorder (PTSD).29 As previously noted, patients who report anxiety after ICU admission also report decreased quality of life. Many patients with post-ICU anxiety had anxiety symptoms that persisted 12 months after discharge.29
Post-traumatic stress disorder. PTSD prevalence after ICU care ranges from 10% to 50%.32,33 Davydow et al33 reported that 40% of ICU survivors developed clinically significant symptoms of avoidance and hyperarousal, occurring twice as frequently as intrusion symptoms (nightmares and flashbacks); this is crucial for accurate assessment of post-ICU trauma symptoms.34 It is important to ask patients if they are avoiding medical appointments, taking alternate routes to avoid driving by hospitals or their doctor’s office, or feeling constantly “on guard” since hospitalization. These post-ICU PTSD symptoms also lower health-related quality of life.35
Predictors of post-ICU PTSD include psychopathology (particularly PTSD or depression) prior to hospitalization and greater ICU benzodiazepine use.32,33 Interestingly, there is a greater risk of PTSD symptoms with higher total benzodiazepine dose rather than prolonged benzodiazepine duration.35 Finally, post-ICU memories of frightening or psychotic ICU experiences are risk factors.32,33 In examining post-ICU PTSD, mechanical ventilation use or duration of use, ICU length of stay, and ICU admission diagnosis have not been shown to be significant risk factors. There is mixed evidence on whether delirium is a risk factor for post-ICU PTSD.32,33
Substance abuse. Post-ICU substance abuse has not been well studied. It is known that alcohol use disorders are independent risk factors for the development of critical illness36 and are associated with an increased risk of mortality in critically ill patients.37 However, there are minimal data outlining alcohol use disorders before and after ICU admission. In examining alcohol use in patients at the time of critical illness and up to 12 months after ICU discharge,38 Davydow et al found a significant decrease in alcohol use from the period just before critical illness to 3 months after ICU discharge. This is not atypical as patients tend to make healthier lifestyle choices and avoid harmful behaviors after critical illness. However, alcohol use significantly increased from 3.8% of the study population at 3 months to 7.5% at 12 months after ICU discharge.38 Many patients with post-ICU alcohol abuse also had unhealthy alcohol use in the year before ICU admission: 80% and 67% of patients with unhealthy alcohol use at 3-month and 12-month follow-up, respectively, exhibited unhealthy alcohol use in the year prior to ICU admission.38
ASSESSMENT AND SCREENING
Several screening tools are used to identify the different aspects of PICS, thereby complicating result comparisons.39–44 Turnbull et al39 examined 425 studies and found 250 instruments used for different measures of ICU survivorship, including physical limitations, cognitive limitations, mental health limitations, participation restrictions, and quality of life. Needham et al40 aimed to minimize heterogeneity through the Core Outcome Measurement Set with the objective of developing a core set of measurement tools for use in all clinical research of acute respiratory failure survivors after hospital discharge (including acute respiratory distress syndrome). Although identification of these measurement tools is a significant advance in consistency in clinical research of symptoms of critically ill patients who have been discharged, caution should be used when implementing these tools in the general ICU survivor population as the study focused only on patients with acute respiratory failure.
Screening tools
Table 140–44 presents screening tools most commonly used for cognitive impairment in PICS patients. Needham et al40 noted that for the “cognitive” outcome group in acute respiratory failure survivors, no instrument reached a priori for consensus; however, the highest rated tool was the Montreal Cognitive Assessment (MoCA-Blind), used to screen patients for neurocognitive symptoms in the post-ICU period. It has been shown to be a reliable screening tool independent of being used for patients who were hospitalized or in the ICU.41 MoCA-Blind uses a cutoff score of 26 to differentiate between normal cognitive function and cognitive impairment; these cutoffs have been found to differ based on patient race and ethnicity.42 It has been recommended to use the traditional MoCA-Blind, excluding the areas with visual elements (visuospatial, executive functioning, and naming portions) to facilitate administering the instrument by phone if needed. The Mini-Mental State Examination (MMSE) has been shown to be a poor measure of cognitive deficits in survivors of acute respiratory failure43 and may underestimate the degree of cognitive impairment compared with other assessment tools that focus on specific cognitive domains.44
Screening instruments for post-intensive care unit cognitive impairment
Table 25,40,45–47 lists commonly used tools for measuring post-ICU emotional dysfunction, including the Hospital Anxiety and Depression Scale (HADS) for detection of anxiety and depression symptoms45 and the Impact Event Scale-Revised (IES-R)46 for assessment of PTSD symptoms. Both the HADS and IESR have been recommended as core outcome measurement sets by Needham et al.40 From a psychiatric perspective, the Patient Health Questionnaire-9 (PHQ-9) is used to screen for depression while the Generalized Anxiety Disorder-7 (GAD-7) is used to screen for anxiety, and the Impact Event Scale-Revised (IES-R) is used to screen for PTSD.
Screening instruments for post-intensive care unit emotional impairment
INTERVENTIONS
There are several intervention strategies for management of cognitive and emotional disturbances. While some treatments are for specific post-ICU impairments, many are useful in managing symptoms spanning multiple domains of PICS. Many critical care units have adopted the ICU Liberation Bundle (Table 3) to prevent delirium, prolonged cognitive impairment, and significant post-ICU psychiatric symptoms.27,28,48 For example, dexmedetomidine has been associated with a lower incidence of delirium compared with other analgesia and sedative agents.49
The ICU Liberation Bundle (A–F)
COGNITIVE REHABILITATION
Prolonged post-ICU cognitive impairment may warrant further investigation. Physical, cognitive, and vocational rehabilitation have been studied in patients with ongoing cognitive dysfunction.31,49,50 In the Returning to Everyday Tasks Utilizing Rehabilitation Networks study,50 cognitive rehabilitation was delivered in the patient’s home once every 2 weeks over a 12-week study period. ICU survivors suffering from post-ICU cognitive impairment who received post-discharge cognitive rehabilitation in addition to “usual” post-discharge care (physical rehabilitation, occupational rehabilitation, nursing care) showed improvement in cognitive function at 3-month follow-up compared with patients who did not undergo cognitive rehabilitation.50 Given variability of cognitive interventions and studied populations, evidence-based recommendations for clinical practice are difficult to determine. There are promising data for the role of aerobic exercise in improving post-ICU cognitive function,12 and neurocognitive testing has been employed for patients with prolonged cognitive impairment.51 However, barriers to assessing cognitive function and thereby providing care to this population include social stigmatization and financial strain, loss of patients to follow-up, and patient frustration over testing performance.51 Of note, there are limited data on the role of these strategies in preventing prolonged cognitive impairment in critical care patients.
MEDICATIONS
Few studies have investigated pharmacologic treatments for cognitive impairment in ICU survivors specifically. Current strategies are from studies of cognitive impairment treatment in patients with traumatic brain injury. Methylphenidate and donepezil have been studied in the traumatic brain injury population and were associated with improvement in memory and attention.52–54 Although these strategies may be considered for ICU survivors with cognitive impairment, they should be implemented cautiously as further investigation is warranted for the critical care population specifically.49 Rosuvastatin was studied in the prevention of delirium and cognitive impairment in ICU patients but was not found to have significant benefit in prevention.55
PSYCHOTHERAPY
Psychotherapy may be beneficial for psychiatric symptom management in ICU survivors. The patient’s presenting psychiatric symptoms may guide the type of therapy recommended. For example, some patients with mild depression, anxiety, or PTSD symptoms may benefit from supportive therapy. Moderate to severe mood and anxiety symptoms may respond more appropriately to cognitive behavioral therapy, while patients with more advanced trauma symptoms may benefit from trauma-based therapy, including but not limited to eye-movement desensitization and reprocessing, a form of psychotherapy that allows patients to access and process traumatic memories through simultaneous focus on external stimuli such as eye movement. Haerizadeh et al reviewed psychological treatment modalities for PTSD in medically ill patients.56 Although limited data were available, 2 of the included trials showed exposure-based cognitive behavioral therapy resulted in a lower incidence of PTSD symptoms compared with control groups. And 3 trials included found eye-movement desensitization and reprocessing to be more effective in reducing PTSD symptoms than relaxation therapy, imaginal exposure, and conventional cognitive behavioral therapy.56
Patients with more severe psychiatric symptoms may warrant pharmacologic management. There is a lack of literature analyzing pharmacologic treatment for depression, anxiety, and PTSD in ICU survivors. It is important to note that ICU survivors warrant ongoing monitoring by a primary care provider or mental health clinician as they may be more sensitive to medication side effects given their underlying medical comorbidities and potential risk of drug interactions with other medications.
ICU DIARIES
ICU diaries are used to fill memory gaps for ICU survivors and provide an understanding of ICU events in a chronologic or narrative account.57 The diaries are often completed by ICU staff including physicians, advanced practice providers, nurses, consultants, and other providers involved in ICU patient care (eg, physical therapists, occupational therapists, music therapists, art therapists). Families may also participate in construction and completion of the ICU diaries.
ICU diaries have been increasingly used as a management strategy for emotional disturbances of PICS in ICU survivors. Data analyzing effects of ICU diaries on psychiatric symptoms in ICU survivors have been mixed. Barreto et al57 found that the use of ICU diaries was associated with decreased rates of depressive symptoms and depression diagnoses, mostly beneficial in ameliorating anxiety symptoms, but did not significantly improve PTSD symptoms. Garrouste-Orgeas et al58 found no statistically significant benefit in reduction of PTSD symptoms from use of ICU diaries in mechanically-ventilated ICU patients at 3-month follow-up.58
Of note, there is no universal template for ICU diaries. This unstructured document is used by the patient and, after discharge, also by the family if the patient so chooses.
THE ROLE OF POST-ICU RECOVERY CLINICS
PICS symptoms after ICU stays led to a re-evaluation of methods of care. Traditionally, within 1 month of hospital discharge, ICU survivors would have a brief follow-up visit with their primary care provider to address the complexities of a potentially extended critical care hospitalization. However, the time constraints of a brief office visit increased the risk that the patient’s complex post-discharge needs would be suboptimally addressed.
Recognizing these issues, critical care providers have developed post-ICU recovery clinics where ICU survivors can receive outpatient follow-up care targeted to their needs. These clinics are composed of multidisciplinary teams usually including but not limited to critical care specialists, physical therapists, case managers, social workers, respiratory therapists, pharmacists, and mental healthcare professionals such as psychiatrists and psychologists.
Reported outcomes of post-ICU clinics have been positive overall in improving depression, anxiety, and PTSD symptoms.59,60 Also, qualitative outcomes have revealed positive results for patients and families, who reported higher levels of satisfaction from involvement in these clinics.60
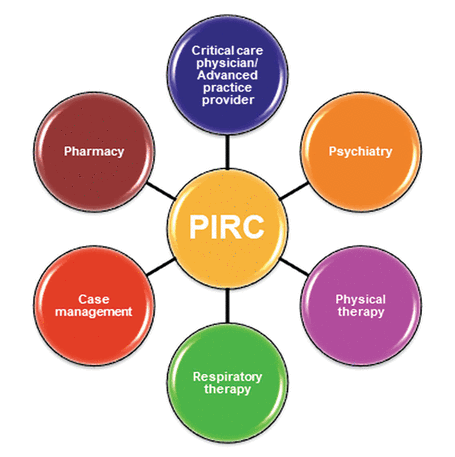
The Post-ICU Recovery Clinic (PIRC) at Cleveland Clinic (Figure 1) involves an ICU physician, ICU advanced practice provider, pharmacist, physical therapist, respiratory therapist, and mental health providers. Patients are triaged as they are discharged from the ICU based on inclusion and exclusion criteria found in Table 4. The patients that meet inclusion criteria are tracked while on the regular nursing floor to capture discharge disposition; patients discharged to skilled nursing facilities are not eligible for the clinic. The PIRC project manager consults with the patient to discuss PICS and the PIRC. If the patient voices interest, a post-discharge PIRC visit is scheduled. The goal is to see patients in the clinic within 4 weeks after hospital discharge.
The multidisciplinary team approach used in the Cleveland Clinic Post-ICU Recovery Clinic (PIRC).
Cleveland Clinic Post-ICU Recovery Clinic: Inclusion and exclusion criteria
During the post-discharge PIRC visit, several screening tools (Tables 1 and 2) are used to determine the patient’s level of physical, cognitive, and emotional impairment. Based on the patient’s symptom severity on screening tools and during personal interviews, a referral may be made to a clinical psychologist for psychotherapy or to a consultation psychiatrist for medication management. If patients show cognitive impairment based on screening or are reporting significant cognitive dysfunction compared with their pre-ICU baseline, a referral is made to neuropsychiatry for further symptom management.
IMPORTANT CHANGES TO MEET A PRESSING NEED
Neuropsychiatric symptoms are prevalent and at times disabling in ICU survivors. Previously, survivors have been at increased risk of psychiatric symptoms going undetected owing to limitations in post-discharge follow-up, mental health stigma, and limitations in financial and social circumstances due in part to acute and chronic medical conditions. ICU survivor neuropsychiatry is an emerging field that continues to be evaluated and is even more pressing in the COVID-19 era.
Clinicians seeing patients in the ICU and in the outpatient setting should be knowledgeable about the potential for PICS and appropriate screening tools for patient monitoring. Even with advances made in identifying screening tools in ICU respiratory survivors, further studies are warranted to evaluate these and other assessments for neuropsychiatric symptoms of PICS across various diagnoses and conditions in ICU survivors.
Another area of continuing research is that of the post-ICU clinic in investigation of long-term outcomes of PICS, including long-term prevalence of neuropsychiatric symptoms, treatment strategies, mortality rates, readmission rates, financial impact for the healthcare system, and patient and caregiver satisfaction. These clinics allow for collaborative care not only in the areas covered in the Cleveland Clinic PIRC but also with geriatric medicine, otorhinolaryngology, endocrinology, nutrition, psychology, neuropsychiatry, and neuropsychology. Establishment of the post-ICU clinics allows clinicians and researchers to further investigate treatment modalities and prevention strategies and to improve care for ICU survivors.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.