A 50-year-old woman with a medical history significant for childhood asthma presented to the emergency department with a 3-week history of worsening dyspnea and cough with bilateral lower-extremity swelling, left-side swelling greater than right-side swelling.
On presentation, her heart rate was 121 beats per minute, blood pressure was 197/133 mm Hg, and respiratory rate was 32 breaths per minute with oxygen saturation of 96% on room air. Physical examination was notable for tachycardia and normal S1 and S2 heart sounds without murmurs, rubs, or gallops. Breath sounds were normal bilaterally. Venous Doppler ultrasonography of the left lower extremity revealed acute distal deep vein thrombosis of the posterior tibial and peroneal veins.
Laboratory evaluation revealed the following:
White blood cell count of 13.1 × 109/L (reference range 3.5–10.5) with neutrophilic predominance
Hemoglobin of 9.8 g/dL (reference range 11.5–15.5)
Platelet count of 588 × 109/L (reference range 150–400)
D-dimer of 2,669 ng/mL (reference range < 500).
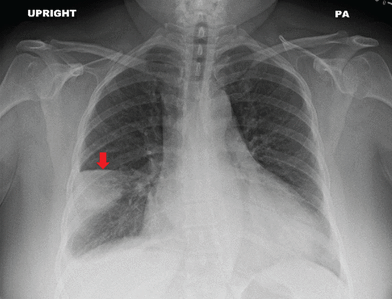
Chest radiography revealed a wedge-shaped opacity in the right lower lobe (Figure 1) concerning for pulmonary infarction. The patient subsequently underwent computed tomographic pulmonary angiography that revealed bilateral segmental and sub-segmental filling defects consistent with acute pulmonary embolism and corresponding opacities in the right and left lower lobes consistent with pulmonary infarction (Figure 2). She was admitted to the hospital, and systemic anti-coagulation was initiated. She ultimately did well and was discharged home. At follow-up 1 month later, her dyspnea had resolved.
Posterior-anterior (PA) view of a chest radiograph demonstrated a wedge-shaped opacity (arrow) in the right middle lobe consistent with Hampton hump.
Contrast-enhanced axial computed tomographic angiography of the chest in a lung window demonstrated a wedge-shaped opacity (arrow) in the right middle lobe.
HAMPTON HUMP AND PULMONARY INFARCTION
Chest radiography is the initial test of choice when evaluating patients presenting with dyspnea because it is inexpensive, widely available, and can be quickly performed at the bedside. A peripherally located wedge-shaped opacity on chest radiography is referred to as Hampton hump (Figure 1), first described in 1940 by Hampton and Castleman,1,2 who performed an autopsy series to demonstrate the site of opacities seen on chest radiography in patients with pulmonary embolism compared with pulmonary infarction seen at autopsy.
Hampton hump is modestly specific for the diagnosis of pulmonary embolism but lacks sensitivity. In a study evaluating radiographs of patients in the multicenter Prospective Investigation of Pulmonary Embolism Diagnosis trial,3 Hampton hump had a sensitivity of 22% and a specificity of 82%. Computed tomographic pulmonary angiography remains the gold-standard for establishing a diagnosis of pulmonary embolism, with a sensitivity of 89% and a specificity of 95%.4
Pulmonary infarction occurs when blood vessel occlusion results in mismatch of oxygen supply and demand and subsequent hypoxia. This triggers a cascade of pathologic processes culminating in tissue necrosis.5 Pulmonary embolism is a common cause of pulmonary infarction, with an estimated annual incidence of 115 per 100,000 people in the United States.6 The true incidence of subsequent pulmonary infarction is variable. In patients diagnosed with pulmonary embolism, pulmonary infarction has been reported in 15% to 31% of patients on follow-up autopsy and in 9% to 36% of patients on computed tomography.5,7,8
The lungs receive a dual supply of oxygenated blood from the bronchial and pulmonary arteries. In cases of proximal pulmonary embolism, pulmonary infarction is not typically seen owing to the presence of dual circulation.9 However, with more distal pulmonary artery occlusions, a sudden influx of collateral blood flow into small-caliber vessels and increased vascular permeability result in intra-alveolar hemorrhage and infarction.5
Pulmonary infarction: Presentation, risk factors, clinical significance
Clinically, pulmonary infarction can present silently or with any combination of chest pain, syncope, cough, and dyspnea. Significant risk factors include smoking, chronic obstructive pulmonary disease, malignancy, shock, and distal small-artery occlusions.5,8 Advanced age has also historically been considered a risk factor, but recent findings suggest younger patients are at highest risk because of a less-evolved collateral system and higher endogenous nitric oxide levels that produce more vascular anastomoses and influx of bronchial flow.10,11
Little is known about the exact clinical significance of pulmonary infarction. According to limited data, mortality and pulmonary embolism recurrence do not significantly differ among patients with acute pulmonary embolism and ensuing pulmonary infarction compared with those without infarction.12 Long-term consequences such as persistent dyspnea, pleuritic pain, postpulmonary embolism syndrome, and chronic thromboembolic pulmonary hypertension are not well known and should be a focal point of further investigation.
In patients presenting with dyspnea, peripheral wedge-shaped opacity on chest radiography should raise suspicion for pulmonary infarction, warranting further evaluation to diagnose pulmonary embolism.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.