ABSTRACT
The authors review the rationale behind and approaches to testing for COVID-19, the quality of currently available tests, the role of data analytics in strategizing testing, and using the electronic medical record and other programs designed to steward COVID-19 testing and follow-up of patients.
WHY SHOULD WE TEST FOR COVID-19?
Diagnostic testing plays a key role in the investigation of any patient suspected of having a novel respiratory viral infection. Timely use of validated, sensitive diagnostics are key to confirming or excluding a diagnosis. The critical importance of testing has never been more apparent than in the current COVID-19 pandemic. Testing patients suspected of being infected with SARS-CoV-2, the virus that causes COVID-19, offers many benefits. For the hospitalized patient, it informs isolation practice, allowing a healthcare system to optimize its use of personal protective equipment (PPE). Without testing, isolation practice would have to be syndromic, resulting in far more isolated patients and a significant increase in the utilization of PPE.
Ready access to testing also informs the strategy for maintaining a robust healthcare workforce and mitigates against the problem of “presenteeism”—the practice of coming to work while ill. Outside the walls of the hospital, identifying infected patients is essential to control the spread of the virus in the community. Diagnosis confirmation makes possible contact tracing by public health authorities who can then identify and isolate others who are ill and quarantine those who are exposed. Testing also provides critical epidemiologic data that help society understand current and future resource needs.1 Finally, the ultimate goal of testing is to identify patients who would benefit from targeted, effective treatment.
WHAT ARE THE COVID-19 TESTS AVAILABLE, AND HOW GOOD ARE THEY?
The US Centers for Disease Control and Prevention (CDC) and state public health laboratories were the only sites with testing available in the early stages of this pandemic in the United States. It became clear that these institutions would be unable to handle the surge of testing that would be associated with the community spread of COVID-19. At this point, the US Food and Drug Administration (FDA) authorized the Emergency Use Authorization (EUA) option to manufacturers and hospital laboratories. This provided two options for laboratories. At the time of this writing, the FDA had approved 60 different EUA assays, most of which were for direct molecular detection, and a minority for serologic testing.2
Laboratories may use an assay that had achieved EUA status or perform the requisite validation studies and submit a SARS-CoV-2 EUA to the FDA for review. The laboratories of the Pathology and Laboratory Medicine Institute (PLMI) at Cleveland Clinic did both.
The initial assay validated for patient testing at Cleveland Clinic was the original CDC SARS-CoV-2 assay, which detects 3 target areas within the nucleocapsid (N) gene. This assay requires a full nucleic acid extraction prior to reverse transcription-and polymerase chain reaction-based nucleic acid amplification. This has been the principle assay used at Cleveland Clinic, and early in the pandemic was performed 24 hours a day, 7 days a week. The results from this test are available within 24 hours from specimen receipt, and often sooner.
The second assay validated was the TIB MOLBIOL/Roche z 480 Assay (TIB MOLBIOL, Adelphia, NJ; Roche Diagnostics, Indianapolis, IN). This assay also requires full nucleic acid extraction prior to reverse transcription- and polymerase chain reaction-based nucleic acid amplification. This assay utilizes a high-quality nucleic acid extract as a substrate and tends to have excellent performance characteristics (eg, a very low limit of detection). These characteristics (ie, well-designed primers and probes and a low limit of detection) usually translate into excellent clinical sensitivity and specificity when high-quality clinical specimens are tested. This assay had not received FDA EUA clearance; therefore, our laboratory performed the studies to achieve this status. The results from this test are also available within 24 hours from specimen receipt, and often sooner.
The need for a rapid assay that could detect SARS-CoV-2 soon became apparent. We investigated three different assays for this purpose; the Xpert Xpress SARS-CoV-2 (Cepheid), the Simplexa COVID-19 Direct Test (Diasorin), and the ID Now COVID-19 (Abbott). Both the Xpert Xpress SARS-CoV-2 and the Simplexa COVID-19 Direct Test passed initial internal validation and were put into use for the rapid detection of SARS-CoV-2. The initial verification studies of the ID Now COVID-19 (Abbott) did not detect some positive specimens; therefore, the implementation of this assay was stopped in Cleveland Clinic’s Ohio hospitals until further studies could be performed.
A challenge with any newly emerging pathogen determining whether a patient is truly positive. An important aspect of accurate testing is appropriate swabbing technique, which includes inserting a swab into the nose parallel to the palate similar to the distance from the nose to the outer opening of the ear (approximately 3-4 inches), then slowly turning the swab for several seconds.3 Similarly important, from the laboratory standpoint, is determining whether the target or analyte is truly present in a specimen. This must first be accomplished to determine the sensitivity and specificity of the tests under consideration. A five-test comparative analysis was performed to assess the sensitivity and specificity of these assays (publication pending). A composite methodology was used to determine if a specimen contained the virus. This method required the specimen to have a positive test result in 2 or more tests for a specimen to be deemed to contain the analyte. A specimen with uniformly negative tests or specimens with only a single positive test were considered to not contain the analyte. A single positive was characterized as a false-positive result because it could not be corroborated by another assay. Our group was particularly interested in false-negative reactions; therefore, the study set was enriched with CDC test-positive specimens.
This analysis demonstrated a greater than 95% sensitivity for the CDC assay, the TIBMOLBIO assay, and the Xpert Xpress SARS-CoV-2. The Simplexa COVID-19 Direct Test and the ID Now COVID-19 produced sensitivities below 90%, which was deemed unacceptable for the testing of inpatients at our facility. We considered the potential utility of the assays with a less than 90% sensitivity in the ambulatory setting wherein the instructions to the patient would not change (eg, symptomatic individuals would be instructed to self-isolate and wear a mask, regardless of the test results) and possibly in settings of very low prevalence, wherein the negative predictive value exceeds 95%, particularly if these were low-risk settings (eg, population screening). Cleveland Clinic laboratories currently offer 4 assays, with one of the platforms performing rapid tests. The availability of numerous platforms allowed expanded testing capacity. Additionally, a fifth option to further increase throughput is currently under evaluation.
The sprint to bring the various COVID-19 tests online in the laboratory concomitantly required the healthcare system laboratory informatics and analytics teams to be agile and, in some cases, creative. Cleveland Clinic’s “go-live” process required an iterative process, with changes to workflow and test definitions in the institutional information systems, as various platforms, methods, and testing priorities were added. This process also required careful synchronization between definitions and processes in the enterprise’s electronic health record and laboratory information system.
When we brought testing in-house near the beginning of the surge of cases, we understood that testing would have to be diversified in order to account for unstable supply lines and lack of committed testing kit allocations. Through examination of current and likely future workflows, three orderable in-house COVID-19 laboratory tests were created: the routine COVID-19 test, the caregiver COVID-19 test, and the rapid-expedited COVID-19 test. This laboratory informatics build was a departure from the typical internal approach but allowed the laboratory to utilize a single test code on multiple platforms. This provides flexibility for the laboratory to shift tests to various platforms based on demand and availability of supplies without changes to the test ordered.
THE IMPACT OF DATA ANALYTICS AND REPORTING ON DECISION-MAKING RELATED TO COVID-19 TESTING
The need to assess test utilization and help guide expansion of testing priority groups across the organization required ready access to data on test volume, turnaround time, ordering location, and test results. Establishing and communicating such data rapidly represented a significant cross-departmental challenge. The laboratory analytics team in conjunction with the Cleveland Clinic Health System enterprise analytics team and information technology reporting team created a number of dashboards that update in real-time displaying the volume of COVID-19 tests performed, both rapid and routine, as well the median turnaround times for these tests. In addition, tests could be further stratified by location (emergency department, ambulatory, inpatient) and by hospital within the health system (Figure 1). In the early stages, these dashboards were iterated almost daily.
COVID-19 test volume dashboard.
The information on these dashboards allowed for more efficient utilization of existing capacity and helped inform decisions regarding the extension of testing to additional patient populations or locations. Furthermore, monitoring turnaround times provided information on ensuring processes were occurring as intended and allowed a method to troubleshoot operational lapses.
WHAT ARE THE IMPLICATIONS OF WHO, WHERE, AND WHEN A PATIENT IS TESTED?
Because of limited testing capacity, most health systems have resorted to prioritizing groups of patients in whom to offer testing based on guidance from the CDC and Ohio Department of Health (ODH).4,5 Priority patients include those with high-risk underlying medical conditions or work-related risk, and those planned to undergo procedures and surgeries. Because many hospitals and procedural areas are observing significantly lower volumes than normal, most are starting the reopening process with regards to nonessential surgeries and procedures. Therefore, considerations regarding testing and logistics are very important. Operationalizing widespread COVID-19 testing can be difficult for a number of reasons, including managing the supply of testing kits and swabbing materials, optimizing PPE availability, and identifying an adequate number of inpatient cohorting units and beds to separate patients with COVID-19-positive, COVID-19-negative, and pending test results. Cohorting is especially important in populations that may not be able to follow social distancing strategies, such as patients with behavioral health problems or dementia. Limitations in items such as swabbing kits led to the Cleveland Clinic Health System pharmacy partnering with supply chain to make universal transport media and pairing into a kit with a swab; as well as 3-D printing facemasks with industry leaders.
At Cleveland Clinic, we took a two-pronged iterative approach to test our emergency department and inpatient populations. For patients admitted through the emergency department, we phased in expedited (rapid) COVID-19 testing based on high-risk patient populations, such as symptomatic intensive care unit patients and labor and delivery patients, and those who needed testing prior to disposition from the emergency department, such as behavioral health admissions. Subsequent phases included testing patients transferring from extended-care facilities, and all admissions to the intensive care units and labor and delivery units (and their companions), as well as symptomatic cancer and immunosuppressed patients. Expedited testing was primarily used in the emergency department because it impacted decisions related to bed placement into cohort areas and to conserve PPE. As the expedited testing supply increased, testing expanded to all admissions from the emergency department in order to identify asymptomatic and presymptomatic patients to allow for cohorting of COVID-19-positive patients and prevent inadvertent spread of disease. Additionally, testing was performed early in the emergency department for those likely to be admitted in order to minimize the impact on the emergency department throughput and PPE use. Broad but targeted testing minimizes risk of transmission to caregivers and other patients, including newborns in the labor and delivery setting.6
This strategy also addresses caregiver concerns about exposure during high-risk procedures such as intubation, noninvasive positive pressure ventilation, nebulized aerosol treatments, and procedures involving the upper and lower airways. Lowering caregiver concern allows for focused patient care, appropriate PPE use and compliance, and optimal training.
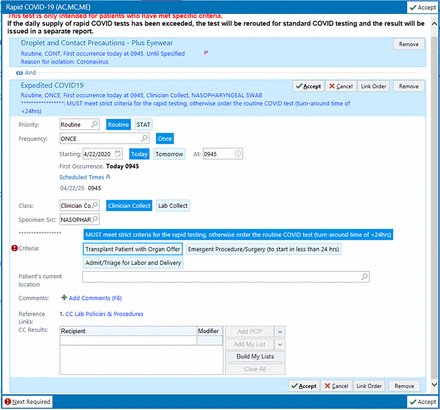
The strategy on the inpatient side was different due to the higher capacity to perform nonrapid COVID-19 testing. Providers are encouraged to utilize testing in symptomatic patients based on clinical judgment without restriction to specific populations. Expedited testing was also available to specific asymptomatic inpatient populations, such as organ transplant recipients with an active organ offer, and patients requiring an emergency surgery or procedure in the next 24 hours (Figures 2 and 3). Testing prior to surgeries and procedures was encouraged in order to understand the risk of complications for the patient and the risk of exposure to the caregivers in the operating room, and ensuring appropriate levels of PPE available in the operating room.7 Considerations when testing inpatients also include ensuring cohorting of patients with status “person under investigation,” moving patients into private rooms when test results are pending, and ensuring adequate PPE is available and being utilized.
Rapid COVID-19 testing inpatient workflow for emergency surgeries and procedures.
Inpatient rapid COVID-19 test order.
Our ambulatory COVID-19 testing strategy and processes evolved quickly over a few weeks. Initially, testing was made available widely without defined criteria, but this quickly overwhelmed our operations and supplies. Additionally, a method for testing patients prior to essential surgery was also implemented. Testing preoperative patients was accomplished in phases, with initial concentration on surgeries and procedures that would pose the greatest risk to patients and caregivers due to aerosolization. COVID-19 testing was also offered to those in the community residing in congregate living facilities, such as extended-care facilities, homeless shelters, group homes, and prisons, as well as for first responders, and as part of cluster investigations. Future testing strategies include wider community testing for symptomatic critical infrastructure workers and asymptomatic individuals.
To coordinate the expansion of testing groups while ensuring consideration of the logistics mentioned above, a COVID-19 Test Stewardship group was created with oversight by Cleveland Clinic Medical Operations, Pathology and Lab Medicine Institute, and Executive leadership. This group brought together the expertise of laboratory medicine physicians, medical operations, supply chain, information technology, nursing, primary care physicians, and continuous improvement teams. This group meets two to three times weekly to develop a strategy for testing, discuss data and operational constraints, as well as implementation and monitoring of initiatives.
HOW DO OUTPATIENTS GET TESTED, AND WHAT IS THE FOLLOW-UP?
Ambulatory COVID-19 testing site considerations
The physical location of COVID-19 testing is a major factor because ensuring the proper infrastructure and processes exist is imperative for both patient and caregiver safety. The considerations include the physical site, ability to test asymptomatic and symptomatic patients and caregivers, availability of supplies (eg, PPE, swabs, testing reagent, eye wash sinks, computer hardware, printers, connectivity), and human resources to support the processes. The physical site should be selected based on patient flow with the least amount of exposure risk. This includes identifying a single entry and separate exit, the ability to have a large enough space to accommodate patient and caregiver flow, and separate spaces for donning and doffing of PPE for caregivers. Exposure risk must be mitigated for the caregivers performing the test and the other patients within the physical space, especially when testing asymptomatic and symptomatic patients in the same facility. The consideration of creating centralizing testing locations also requires review of PPE inventory and testing supplies across the entire organization, and ensuring there is adequate amount to support overall patient care. Human resources are key to the successful launch of a short-term testing site at the onset of the pandemic period. Due to changes in routine operations where several outpatient locations experienced lower volumes in the Cleveland Clinic Health System, resources were available to support a free-standing testing site. These caregivers were trained to perform the swabbing in a highly reliable and safe manner.
A long-term testing site strategy must be integrated into normal clinical operations. The same considerations noted above must be incorporated in order to ensure a successful transition. This planning involves identifying a space for testing at various locations that are scattered across a geographic area for patient convenience. Whether swabbing can be performed by patients themselves with clinician monitoring should be investigated in order to conserve PPE and optimize staffing as personnel are reallocated to their primary sites of care. The testing of saliva is another option that is currently under investigation. Although early morning saliva specimens appear to be an adequate alternative specimen for COVID-19 testing, this may not be operationally timely when a patient presents ill to their care provider. We will, therefore, compare saliva co-incidentally collected with a nasal-nasopharyngeal swab to assess the real-world comparability of this specimen type.
Streamlining COVID-19 testing through the COVID-19 hotline
A Cleveland Clinic COVID-19 Hotline was created in response to the overwhelming demand for testing at the beginning of the pandemic. The testing site experienced a large influx of ordered tests with logistical issues arising without a structured operational process. Large crowds arrived at the site to receive testing because appointments were not scheduled. This crowding resulted in long wait times, high levels of frustration for patients and caregivers, and concerns about viral transmission at the site. The surge in tests ordered raised questions about the appropriateness of ordering in the setting of limited testing capacity. This was balanced with a desire to prioritize testing for symptomatic caregivers.
The COVID-19 Hotline was created to address these concerns and priorities and serves two basic purposes: to provide secondary screening for all patients prior to COVID-19 testing, and to provide immediate availability and testing for symptomatic caregivers. Hotline leaders worked together with outpatient providers, infectious disease specialists, the scheduling team, and the information technology team to ensure standardization of the secondary screening and test ordering process. Screening criteria were continuously updated to align with CDC guidelines so that the secondary screening provided by the Hotline was standardized and objective. A call center was created for the Hotline, staffed by an advanced-practice provider for 16 hours a day, 7 days a week, to accommodate both the secondary screening process and caregiver phone calls.
After the primary screening of symptomatic patients by an outpatient provider, the patient is referred to the Hotline through the EHR for secondary screening. If the patient meets the standardized secondary screening criteria by chart review, the Hotline advanced-practice provider places the COVID-19 test order. These orders are then routed to a team of schedulers, and patients are scheduled for an appointment time to get tested in order to avoid long wait times (Figure 4). A similar process is also used for symptomatic caregivers to provide immediate access to a care provider and testing. The caregiver calls the Hotline phone number and speaks with an advanced-practice provider, who assesses and reviews the symptoms and subsequently orders the test as indicated. Appointment scheduling proceeds as outlined after secondary screening. Establishment of the Hotline achieved the two primary goals through a coordinated, team-based effort.
COVID-19 testing process for symptomatic outpatients.
COVID-19 home monitoring program
Patients are automatically enrolled in the Cleveland Clinic COVID-19 home monitoring program following a positive test result, an ambulatory clinician virtual assessment, or after hospital discharge for COVID-19. All newly identified patients receive an outreach call with instructions on home isolation, education about COVID-19, provider screening for concerns about social support and home safety, and an invitation to engage with the MyChart Care Companion, a patient-engagement platform available on smartphone and web-based platforms (Figure 5). Based on CDC guidelines, Cleveland Clinic partnered with Epic to custom-build a COVID-19 care plan in the platform in order to optimize engagement.
Epic MyChart care companion smartphone app.
Patients in the home monitoring program are monitored daily for 7 days after discharge from the hospital or for 14 days after symptom onset if they entered the program as an ambulatory patient.
Daily monitoring consists of telephonic outreach from a registered nurse or allied health professional and a self-monitoring program in the MyChart Care Companion app that allows for patient-entered data of COVID-19 symptoms. Using either or both methods, patients are asked whether any of a list of symptoms are present, and whether those symptoms are getting better, getting worse, or staying the same. Symptoms include cough, dyspnea (“Have you been able to perform your usual activities without shortness of breath?”), weakness, vomiting (“Have you been able to keep down fluids?”), diarrhea, and appetite, as well as pulse oximetry and temperature, if available.
Patients reporting new or worsening symptoms via the app see a message stating that their symptoms are going to be forwarded to a clinician. A list of enrolled patients is organized in a registry within the EHR with data populated via the home monitoring program. A pool of nurses and clinicians monitors the EHR registry and flags symptoms that are worsening. After a nursing assessment, a patient may then be escalated for additional care with two options: an urgent virtual evaluation with the patient’s primary care provider or a “virtualist” physician on call, or referral to the emergency department with direct handoff communication. The primary care or virtualist physician may order additional medications (eg, cough medications, bronchodilator), arrange for additional diagnostic testing (eg, laboratory, radiography) in a designated facility that can manage COVID-19-positive patients, or order mobile testing by a visiting paramedic or allied health professional. For patients who do not desire escalation of care and who instead choose to focus on comfort, palliative care is activated through a virtual visit assessment with urgent initiation of services if a patient reports worsening symptoms. Palliative care measures are consistent with Cleveland Clinic’s ethical framework and those reported in other countries.8 Throughout the process, the patient’s primary care provider or primary specialty consultant is apprised of all outreach via the EHR.
Enabling the electronic health record to help steward COVID-19 testing
Part of the success of the testing program was due to the recognition that health information technology aspects are a critical part of the overall testing strategy.9 Leadership from the Cleveland Clinic information technology division was engaged early in the planning process to help ensure that the full potential of the EHR to support testing needs was realized. A vital component of this planning was ensuring that there was representation for information technology as part of the overall strategic command center as well as part of all the operational task forces and work-stream groups.
As the testing recommendations were and are in flux, we developed ordering processes to allow for rapid changes to meet the ever-changing workflows. A variety of techniques were utilized to provide clinical decision support at the point of ordering, including embedding the standard COVID-19 order in unique testing panels so that the order could be made accessible only to certain users and departments. To this end, several required and non-required questions were included in all testing orders to guide proper testing utilization and alignment with identified testing criteria, with different questions viewable for the different use cases. For example, the criteria options provided for a patient in an emergency department setting are not the same that are seen in the inpatient or ambulatory version of the order. Inpatient orders were paneled with the correct isolation precaution orders, which were preselected.
Discrete information in the EHR was also utilized to present the correct order to the end-user. This was particularly useful to ensure that the correct order was placed for caregivers, which enabled prioritization and downstream tracking by the occupational health department. Orders and results were also used to populate visualization tools in the patient chart, schedules, and patient lists as well as dashboards and other reporting tools. Audit reports were created to track information such as ordering user, location, department, reason, and test result to ensure that testing was being used as indicated.
Some of the challenges with the information technology aspects were the various laboratory information systems in use throughout the Cleveland Clinic Health System. Extensive testing was required to ensure that all orders were received by the various laboratory information systems and that results were correctly filed back into the EHR in a standardized fashion. Changes made to streamline a particular testing workflow, such as scheduling, would very easily alter another workflow such as ordering in the emergency department. Extensive discussions were necessary to work through all the nuances for the creation of new orders or modifications to any existing orders.
Finally, caution must be taken to not put the burden of enforcement on the EHR to ensure adherence to testing best practices and guidelines. Overly complicated or restrictive build, including interruptive alerts, can have unintended consequences, such as contributing to alert fatigue, deterring appropriate use of available testing, and making the system unusable. Importantly, defects in workflows are seldom overcome by technology controls.
CONCLUSION
Operationalizing testing for COVID-19 is a major endeavor and requires thoughtful planning. Planning must take into account a number of logistical items, including test accuracy, appropriate patient groups, using data to drive decision-making, supply chains, physical testing locations, and patient follow-up. This complex process should also include expertise from a broad range of fields such as lab medicine, operations, infectious disease, supply chain, nursing, data analysis, and information technology to ensure success.
Footnotes
The statements and opinions expressed in COVID-19 Curbside Consults are based on experience and the available literature as of the date posted. While we try to regularly update this content, any offered recommendations cannot be substituted for the clinical judgment of clinicians caring for individual patients.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.