ABSTRACT
Numerous immunomodulating agents are currently being studied in clinical trials for the treatment of COVID-19, including interferon therapies. Interferons are naturally occurring host antiviral proteins upstream of the inflammatory pathway that are released by host cells in response to the presence of viral pathogens. It is known that beta coronaviruses deploy anti-interferon defenses to escape host innate immunity early in the infection course, and thus interferons have become attractive candidates for treatment of COVID-19. Questions surrounding timing, type of interferon, and route of administration all remain unanswered. Here we discuss the role of interferons in host antiviral immunity, and review the current data surrounding use of interferons in COVID-19.
INTRODUCTION
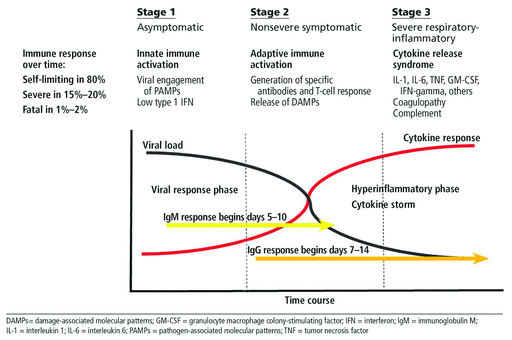
The current pandemic of COVID-19 has created an unprecedented race in biotechnology in a search for effective therapies and a preventive vaccine. To model the therapeutic landscape for COVID-19, we think it is useful to employ an idealized model of disease progression (Figure 1) that envisions an initial phase (stage 1) in which SARS-CoV-2 engages the innate immune system, generally in the upper respiratory tract, which can in theory dispatch the pathogen before productive and progressive infection is established. In most cases, however, this does not occur, leading to stage 2, in which adaptive immunity is deployed, generating a specific antibody response as well T-cell-mediated immunity. By a wide margin, the majority of patients will resolve their COVID-19 disease without further sequelae and then establish immunologic memory to prevent reinfection.
Three stages of COVID-19 disease.
The third phase of the disease (stage 3), which has been variably referred to under many labels including cytokine storm or cytokine release syndrome, occurs predominantly in those who have well-described comorbidities, who then experience uncontrolled inflammation that potentially leads to progressive pulmonary disease and death.1 To date, most COVID-19 therapeutic research has focused on searching for immune-based therapies to deal with the cytokine-mediated inflammatory manifestations (stage 3), a review of which was previously presented in this journal.1
A second front has been the search for agents that will act in stage 1 or 2 and directly limit the infection at the earliest time point, with a goal of avoiding progression to stage 3 with its hyperinflammation and progressive pulmonary and other target organ damage. A variety of direct antiviral agents are already in clinical trials, and one such agent, remdesivir,2 has already shown some promise and has been approved by the FDA for use in hospitalized patients. Numerous other candidate antiviral agents and other antiviral strategies, including convalescent plasma and monoclonal antibodies, are also under development.
This review will focus on a third front of therapeutics, namely the use of interferons, a family of naturally occurring host antiviral proteins that potentially can enhance viral clearance, with or without synthetic antiviral agents. Interferons are already in use to treat numerous diseases, including viral infections such as hepatitis B and C as well as autoimmune diseases such as multiple sclerosis, and in clinical trials in a host of other disorders.
A PRIMER ON INTERFERON BIOLOGY AND ITS ROLE IN ANTIVIRAL DEFENSE
Humans have been afflicted by viruses throughout our evolutionary history, and our immune system has evolved with them in our effort to defend ourselves and preserve our species. The integrated immune system we have developed is a complex network involving both innate and adaptive limbs evolved to defend us without harming us. Prominent within this system are the interferons.
Interferons are cytokines made and released by host cells in response to the presence of viral pathogens. There are three families of interferons: type I, type II, and type III (Table 1).
Currently available interferons
Type II interferon will not be discussed further in this brief review, for while it has antiviral properties, it is considered most important for its immunostimulatory and immunomodulatory effects, being produced by both cells of innate immunity and cells of adaptive immunity, especially T cells.3
Type I interferons encompass a large family of molecules including 13 members of the alpha family and a single member of the beta family (ie, interferon beta). Other forms of type I interferon have also been described, though our knowledge of their biologic role remains limited. Type I interferons are produced by both hematopoetic and viscerosomatic cells but especially by plasmacytoid dendritic cells. Type I interferons bind to the interferon type I receptor, which is widely expressed throughout the host.
Type III interferons, also referred to as interferon lambda, also play a prominent role in antiviral defenses.4 There are four known members of this family, and they bind to their own cognate dimeric receptors that, in contrast to type I interferon receptors, have limited expression, being found enriched on epithelial cell surfaces.
Type I and III interferons are induced by interactions between viral elements and a number of pathogen recognition receptors in the cytoplasmic or endosomal compartments (ie, Toll-like receptors, RIG-I [retinoic acid-inducible gene-I]-like receptors, others), leading to a complex cascade of intracellular signaling events culminating in the transcription of interferons, as well as the induction of the inflam matory response. Both type I (alpha, beta) and type III (lambda) interferons then can be released into the surrounding tissue, where they can activate cells bear ing their cognate receptor.3,4 Because type I interferon receptors are widely distributed, type I interferons can active both hematopoietic and viscerosomatic cells in an autocrine and paracrine fashion, whereas type III interferons are more restricted, but not limited, to epithelial cells.
Once bound to their receptors, interferons mediate their activity via activation of JAK-STAT (Janus kinase-signal transducer and activator of transcription protein) signaling pathways in combination with other cellular elements (interferon regulatory factors, or IRFs), leading to induction of hundreds of interferon-stimulated genes to achieve a cell-intrinsic state of viral resistance.
The interferon pathway with its specific role in viral defense and integration into the immune response is ancient in terms of immune evolution, dating back over 450 million years and entering the phylogenetic tree at the stage of jawed fish.5 These defenses have placed viruses under intense evolutionary pressure, and they have accordingly developed a countless array of countermeasures to evade the defenses.
SARS-CoV-2 and interferon pathways
There is now robust evidence based on preclinical and clinical investigations6,7 demonstrating that beta coronaviruses, including SARS-CoV and SARS-CoV-2, can deploy a series of molecular anti-interferon defenses, enabling them to escape innate immunity early in the course of the infection. The ramifications of this are profound, for in the earliest phase of infection, antedating the development of an adaptive humoral and cellular response, interferon is critical in limiting viral replication and spread. Both SARS-CoV and SARS-CoV-2 have been demonstrated to suppress type I and type III interferon responses. Recently the molecular nature of this suppression has been localized to a single gene product of the SARS-CoV-2 virus.8 Preclinical models of respiratory infections with SARS-CoV and MERS-CoV have demonstrated the importance of the interferon system in host protection as well as the effectiveness of interferon therapies.4
Two important studies have given further strength to the centrality of the role of interferon in host defense in COVID-19, with one demonstrating that a small percentage of patients with life-threatening infections harbor genes reflecting inborn errors within the type I interferon pathway,9 and the second demonstrating that approximately 10% of patients with life-threatening COVID-19 have immunoglobulin G autoantibodies directed against interferon type I.10 Collectively these studies strongly suggest that deficits, both genetic and acquired, within interferon pathways contribute to COVID-19 morbidity and mortality and provide impetus to explore ways to deliver interferon effectively as a therapeutic strategy.
THE DARK SIDE OF INTERFERON
On the other hand, interferons also can contribute to local and systemic inflammation and induce off-target tissue damage. Evidence for this in COVID-19 includes the detection of type I interferon gene expression in classical monocytes from patients with advanced but not mild COVID-19 infection as well as from the lung and bronchial alveolar lavage fluid of patients with advanced disease.11 Thus, a delayed surge of interferon or a sustained presence of interferon can theoretically exacerbate the inflammatory response in phase 3 of our idealized model (Figure 1), and therefore therapeutic use of interferons must be weighed very carefully in terms of type of interferon employed, as does timing and route of administration.
TYPE I OR TYPE III INTERFERONS AS POTENTIAL THERAPY FOR COVID-19
There is interest in utilizing both type I and type III interferons therapeutically and prophylactically in the treatment of COVID-19. As of November 2, 2020, there were 23 studies of various formulations of type I interferon (6 of interferon alpha, 17 of interferon beta), and 5 studies of type III interferon (interferon lamba) registered on clinicaltrials.gov (Table 2). Several of these studies use modified versions incorporating pegylation and routes of administration that include parenteral and aerosol formulations. Interferon lambda has gained increasing interest because it is active locally and regionally, as its cognate receptor is expressed primarily but not exclusively on epithelial surfaces.12 Accordingly, it is postulated that the feared systemic inflammatory effects of type I interferon, which is active systemically, will be abrogated.
Clinical trials involving type I and III interferons in COVID-19
Studies of interferons to date
Interferon alpha has been used extensively to treat viral hepatitis (Table 1). Its side effects are well known and often led to cessation of therapy or dose reduction during the interferon era of hepatitis C treatment. Short-term side effects occurring during the first weeks of treatment include flu-like symptoms such as fever, chills, headaches, arthralgia, and myalgia. Other interferon-related side effects include bone marrow suppression, especially neutropenia, and neuropsychiatric symptoms including depression and fatigue, which led to significant quality-of-life impairment in treatment of chronic hepatitis C. Severe depression and even suicide have been reported.13 Treatment with interferon alpha can also induce autoimmune phenomena, most commonly autoimmune thyroiditis, but this is encountered in the setting of chronic administration and would be unlikely to be encountered with short-term administration.14
Interferon beta has been approved since 1996 for treatment of multiple sclerosis, and major side effects include injection site reactions, fever, and headache. Interferon lambda is not yet approved but has completed phase 2 trials in hepatitis B, C, and D and is currently in phase 3 trials for treatment of chronic hepatitis C infection.
Published data on use of interferon in the treatment of COVID-19 are limited. In an open-label randomized phase 2 trial,15 patients with mild to moderate COVID-19 were randomly assigned to receive a 14-day combination of lopinavir-ritonavir, ribavirin, and 3 doses of interferon beta-1b (8 million IU) on alternate days (n = 86) or 14 days of lopinavir-ritonavir (n = 41). The median time from symptom onset to start of study treatment was 5 days. The combination-treatment group had a significantly shorter median time to negative nasopharyngeal swab (7 days [inter-quartile range 5–11]) than the control group (12 days [8–15]; hazard ratio 4.37, 95% confidence interval [CI] 1.86–10.24], P = .0010). No patient died, and there were no safety concerns.
In another randomized control trial, Davoudi-Monfared et al16 evaluated interferon beta-1a in severe COVID-19: 42 patients received subcutaneous interferon beta 1a in addition to the national protocol in place in Iran, where the trial was conducted (hydroxychloroquine plus lopinavir-ritonavir or atazanavir-ritonavir). The interferon dose was 44 µg/mL (12 MIU/mL) injected subcutaneously 3 times weekly for 2 weeks. The control group received the national protocol. The study group did not have a shorter time to clinical response compared with control (9.7 vs 8.3 days respectively, P = .95), but did have a higher discharge rate by day 14 (66.7% vs 43.6%, odds ratio [OR] 2.5, 95% CI 1.05–6.37) and a lower mortality rate at 28 days (19% vs 43.6% respectively, P = .015). Early administration significantly reduced mortality (OR 13.5, 95% CI 1.5–118).
Concerning interferon alpha studies, only 1 study (nonrandomized, not peer-reviewed), by Zhou et al,17 compared treatment with nebulized interferon alfa-2b or arbidol (an antiviral medication used for influenza in Russia and China), or a combination of the 2, in a retrospective cohort of 77 adults with moderate COVID-19 in China. A shorter time to viral clearance from the upper respiratory tract and reduction in systemic inflammation was seen in the interferon alfa-2b group with or without the antiviral agent. However, participants in the interferon alfa-2b group were younger and had fewer comorbidities than those in the arbidiol group. The nebulized interferon alfa-2b formulation is not approved by the US Food and Drug Administration for use in the United States.
A pre-peer-reviewed systematic review of MEDLINE and MedRxiv studies of select immune-based therapies that included type I interferons revealed significantly lower odds of death (OR 0.19, 95% CI 0.04–0.85, P = .03) for recipients of type 1 interferon, but the conclusions are seriously limited by the lack of available randomized controlled studies and inclusion of non-peer-reviewed publications.18
Collectively these data demonstrate promise for interferon therapy as an adjunct in the treatment of COVID-19 particularly when given early in the course of the infection. However, as has been seen many times thus far in the pandemic, promising results from small preliminary trials of numerous agents have failed when tested in large randomized controlled clinical trials. Caution is clearly warranted.
Of note, a phase 1b study of a monoclonal antibody that depletes plasmacytoid dendritic cells that could potentially lead to decreased type I interferon production is currently recruiting patients hospitalized with COVID-19 pneumonia (with hypoxia, lymphopenia, and elevated markers of hyperinflammation) (NCT04526912).
CONCLUSION
Interferon therapies for COVID-19 have a real potential to contribute to the management of COVID-19 but issues of timing, type of interferon and route of administration all remain unanswered. There is also potential to combine interferons with other more traditional antiviral therapies but this remains unproven. Exploiting the antiviral properties of type I or type III interferons while avoiding potential toxicities related to hyperinflammation is critical to establish for their success to be realized.
DISCLOSURES
Dr. Leonard Calabrese has disclosed financial relationships (consulting, teaching, or speaking) with Abbvie Pharmaceuticals, BMS, Crescendo, GSK, Genentech-Roche, Horizon Pharma, Janssen, Novartis, Pfizer, Regeneron, Sanofi Aventis, and USB. Dr. Lenfant reports no relevant financial relationships which, in the context of their contribution, could be perceived as a potential conflict of interest. Dr. Cassandra Calabrese has disclosed financial relationships (consulting, teaching, or speaking) with Abbvie and Sanofi-Regeneron.
Footnotes
The statements and opinions expressed in COVID-19 Curbside Consults are based on experience and the available literature as of the date posted. While we try to regularly update this content, any offered recommendations cannot be substituted for the clinical judgment of clinicians caring for individual patients.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.