ABSTRACT
The home test kits for detecting SARS-CoV-2 infection with Food and Drug Administration emergency use authorization primarily use either isothermal nucleic acid amplification or antigen detection, and each test has advantages and limitations in terms of sensitivity and specificity, cost, results reporting, and results turnaround time. In clinical studies, these tests provide accurate positive results in symptomatic individuals, although negative results are less accurate. There are also accuracy concerns for positive results in asymptomatic individuals. These factors have implications for their clinical interpretation and use.
INTRODUCTION
Identifying individuals infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a focal point throughout the coronavirus disease 2019 (COVID-19) pandemic, creating a public health need for timely, reliable, and readily available COVID-19 testing. However, there have been significant and ongoing issues with obtaining the testing supplies needed to address the demand, making it necessary to limit testing.1 The recent FDA emergency use authorization of COVID-19 tests that can be conducted at home will substantially expand the ability of individuals to obtain a test and to receive the results quickly.
When this article was published, 3 home COVID-19 tests had been authorized. The tests use either isothermal amplification or antigen detection. Clinical laboratories have substantial experience with both methods. Herein, we review the advantages and limitations of these tests with an emphasis on the appropriate interpretation of test results. Table 1 shows an overview of the tests.
Commercially available home COVID-19 tests
ISOTHERMAL AMPLIFICATION TEST
Isothermal amplification uses enzymatic means, such as helicase or other enzymes, to separate the 2 strands of DNA rather than heat. In contrast, reverse transcription polymerase chain reaction (RT-PCR) tests use sophisticated thermal cycling equipment for this amplification. Isothermal amplification is usually done without a nucleic acid extraction step. Omitting nucleic acid extraction and eliminating the need for thermocycling made it possible to adapt this technology for at-home and point-of-care testing.
The Lucira COVID-19 All-In-One Test Kit (Lucira Health, Emeryville, CA) is the only isothermal amplification assay with FDA emergency use authorization for home use. It uses self-collected nasal swab samples. This test requires a prescription and has been cleared for use on symptomatic individuals either at home or in a point-of-care setting. Healthcare providers are required to report test results to public health authorities.
This assay targets 2 different portions of the N gene of the SARS-CoV-2 virus, and it has 2 internal controls to assess the appropriate functioning of the assay. It claims a limit of detection of 2,700 genome equivalents/swab (per reaction), which is moderately sensitive compared with most laboratory based RT-PCR assays. The submission data had results on 101 specimens from symptomatic individuals, 51 of whom were infected as determined by RT-PCR testing.2 The submission claimed a 94.1% positive percent agreement across all cycle threshold values and a 98% negative percent agreement when compared with control testing. It had 100% positive agreement after eliminating samples with low levels of virus that no longer reflected active virus. Other manufacturers of isothermal amplification tests are also working toward producing at-home testing products.
Note: Cycle threshold or Ct values represent the point in the PCR reaction wherein the fluorescent signal of a positive reaction exceeds a set limit or threshold. This value is inversely proportional to the amount of target (ie, virus in this example) present in the original specimen. Therefore, an early or low cycle threshold value correlates with a greater quantity of virus, whereas a late or greater value correlates with a small amount of virus in the original specimen.
ANTIGEN DETECTION TESTS
Antigen detection tests for infectious agents have been used for many years, and the performance of these assays is well understood. Group A Streptococcus tests are likely the most widely recognized antigen detection tests by healthcare providers and the public. Knowledge of the performance characteristics of these assays facilitates an understanding of the strengths and limitations of SARS-CoV-2 antigen tests, as there are many parallels. The limited sensitivity of antigen detection assays compared with nucleic acid amplification assays has been well documented for other respiratory viruses, such as influenza and respiratory syncytial virus.3,4
The intermolecular interactions that underlie these tests are the antigen-antibody interactions. A simplified explanation is that an antibody manufactured specifically for a particular antigen is used to detect the pathogen that expresses that antigen. The detection methods range from colorimetric detection that can be interpreted visually (eg, a positive pregnancy test) or via a spectrophotometer to advanced fluorometric or chemiluminometric reactions that can be detected by an instrument. Results detected by instrumentation may provide increased sensitivity, improved reliability (ie, more objective and less subjective interpretation), and the opportunity for electronic reporting to patients, healthcare providers, and even public health officials. These assays include processing and reaction control material to ensure the validity of the result.
Ellume COVID-19 home test
The first antigen detection test to receive FDA emergency use authorization for home use was the Ellume COVID-19 Home Test. This assay detects the SARS-CoV-2 nucleocapsid antigen. It has been cleared for testing nasal midturbinate specimens from both symptomatic and asymptomatic individuals aged 2 years of age and older, although individuals younger than 16 years need to have the specimen collected by an adult. Significantly, this test is available over-the-counter without a prescription. A nasal swab is collected, processed according to simple instructions, then the detection device wirelessly displays the results via a smartphone app that also reports the results to public health departments in a manner that complies with the patient data protection established by Health Insurance Portability and Accountability Act (ie, HIPPA compliant).
The Ellume test received FDA authorization based on data from 198 specimens, 37 of which were shown to contain the SARS-CoV-2 by RT-PCR testing. Results showed that this antigen detection method detected 35 of the 37 positive specimens (94.6% positive percent agreement). Also, there were 5 false-positive reactions, all of which occurred in asymptomatic individuals.5
BinaxNOW COVID-19 Ag card home test
The second antigen detection test authorized for home use is the BinaxNOW COVID-19 Ag Card Home Test. This test combines the BinaxNOW COVID-19 Ag Card, which is currently used in healthcare settings, with a smartphone app and eMed, an internet electronic care delivery service. The eMed service determines the patient’s eligibility, guides the patient through specimen collection, and uses a HIPPA-compliant smartphone app to handle public health department reporting requirements.
The submission data reported to the FDA included both home performance and performance of the test card by healthcare professionals. The home test performance trial included data on 52 individuals who were within 7 days from the symptom onset, 24 of whom were positive for SARS-CoV-2 by RT-PCR testing. When all cycle threshold values were considered, the test had an overall 91.7% positive percent agreement and a 100% negative percent agreement with the RT-PCR tests. This performance increased to 100% positive percent agreement for those with cycle threshold values of 33 cycles or less, indicating they were early in the disease course.6
Data on test card performance in non-home use (ie, performance of the test by healthcare providers) included 460 patients from 10 clinical sites. The overall performance for those 7 days or fewer from the onset of symptoms for all cycle threshold values showed an 84.6% positive percent agreement and 98.5% negative percent agreement. The performance improved to 95.6% positive percent agreement when those 7 days or fewer from the onset of symptoms with cycle threshold values of 33 cycles or less were considered.6
ADVANTAGES OF HOME COVID-19 TESTING
An important aspect of home tests for COVID-19, which are relatively simple to perform and interpret, is the immediacy of test results, between 15 and 30 minutes. Previously, patients had to wait several days or more for results from commercial reference laboratories.
Some home COVID-19 tests have smartphone apps that record the results, 2 of which have mechanism for reporting results to public health departments. This reporting feature should be required for all current and future home COVID-19 tests, given the importance of public health interventions to overcome this pandemic. Test users should be aware of state laws requiring providers to report certain test results, such as positive SARS-CoV-2 results, to public health authorities.
We compliment the efforts by manufacturers and the FDA to bring forward additional COVID-19 tests, given the history of limited access to timely test results for many patients. And we compliment the Centers for Disease Control and Prevention (CDC) for its guidance regarding test result interpretation.7
Additional advantages of home COVID-19 testing include the ability for individuals to test themselves without traveling to a healthcare facility and the preservation of personal protective equipment. When a potentially infected individual travels to a facility, there is always a danger of the infection transmission to patients, visitors, and to the healthcare providers who have to obtain the specimen. In turn, this preserves the personal protective equipment for use elsewhere.
DISADVANTAGES OF HOME COVID-19 TESTING
There are several important issues regarding the use and interpretation of home COVID-19 tests. Foremost among these is obtaining a quality specimen and the performance of the test. The process for the consumer has been simplified by the manufacturer-provided visual aids, videos, or online guidance to assist in specimen collection and understanding test performance. For the home use emergency use authorization submission, the FDA required feasibility data showing that people in the authorized age ranges can safely and accurately perform these tests. It is important that individuals performing these tests read and follow manufacturer’s instructions. Regardless of the test type, whether performed at home or in the laboratory, an inferior quality specimen often translates into inferior test results.
HOW ACCURATE ARE HOME TESTS?
Two significant issues for home COVID-19 tests involve the sensitivity and specificity of the assays and, depending on the disease prevalence in any given catchment area or patient population, the resultant positive-predictive values of the test in asymptomatic individuals in whom pretest probability is lower on average. Sensitivity can be thought of as the test’s ability to detect what it is designed to detect (ie, positive result). The limit of detection is a parameter important for determining the analytical sensitivity of the assay. The lower the limit of detection, the higher analytical sensitivity.
Isothermal amplification techniques often exclude a nucleic acid extraction step prior to the amplification of the nucleic acid target. The process of nucleic acid extraction provides a more purified substrate for nucleic acid amplification by removing cellular debris and other extracellular material (eg, mucous) that may inhibit the amplification reaction.8 The exclusion of this step is one reason for the potentially lower sensitivity of isothermal amplification reactions compared with traditional RT-PCR tests that follows nucleic acid extraction.9,10 The limit of detection has been compared for various SARS-CoV-2 assays using the FDA SARS-CoV-2 reference panel.11 Although home COVID-19 tests are not included in this assay comparison, the limit of detection for the direct isothermal amplification assays that are included are not as low as those assays that use nucleic acid extraction and traditional RT-PCR tests.
The second significant issue with these tests involves their performance in asymptomatic populations and the potential generation of false-positive reactions. To add to the challenge of result interpretation in an asymptomatic individual, since COVID-19 viral burden typically peaks before or coincident with the manifestation of signs and symptoms (presymptomatic cases) and that asymptomatic infections are not uncommon, a positive result from an asymptomatic individual with exposure to a suspected or known case cannot always be viewed as false-positive and, thus, should be investigated further. In short, a positive result in an asymptomatic individual could be either a true-positive or a false-positive, so confirmatory testing is necessary.
HOW DISEASE PREVALENCE AFFECTS TESTING RESULTS
Even a highly accurate test can perform differently with respect to its positive-predictive value based on the prevalence of disease in the population being tested. The positive-predictive value is the likelihood that a positive test result truly indicates the presence of disease in the individual being tested. It is influenced by two factors:
test performance characteristics, and
pretest probability of the disease being present in the individual.
Of note, the disease prevalence in the population is factored into the pretest probability. This is important because in a high transmission-intensity setting where most transmissions occur via asymptomatic or presymptomatic spread, the pretest probability is highly influenced by the viral load (ie, cycle threshold values) rather than symptomatology.12,13 Therefore, disease prevalence should be factored in when interpreting COVID-19 antigen test results.
The analytical test performance characteristics that influence the predictive value of a test can be determined using the test’s sensitivity and specificity, and it is termed “likelihood ratio.” Factors that influence the pretest probability include the clinical impression (ie, signs, symptoms, and clinical history) of the patient being tested, as well as the prevalence of the disease in the community, as noted above. For example, if someone develops fever and body aches during the hot days of summer, the disease is likely caused by something other than influenza, since the influenza virus is not prevalent at that time. In contrast, influenza would be the most probable cause of such symptoms in the winter months when the influenza virus is prevalent.
The interaction between the pretest probability and the likelihood ratio of a test were eloquently explained by TJ Fagan in a 1975 paper that introduced Fagan’s nomogram.14 These concepts, which are crucial to the interpretation of COVID-19 tests, were succinctly explained by A Prinzi with respect to testing for SARS-CoV-2.15
TESTING ACCURACY IN SYMPTOMATIC INDIVIDUALS
There is a high pretest probability that a test for SARS-CoV-2 will be positive when we test specimens from individuals who have signs and symptoms of COVID-19 in the midst of an ongoing pandemic. The currently available home COVID-19 tests, based on the reported test performance characteristics, should perform well regarding positive results in symptomatic individuals.
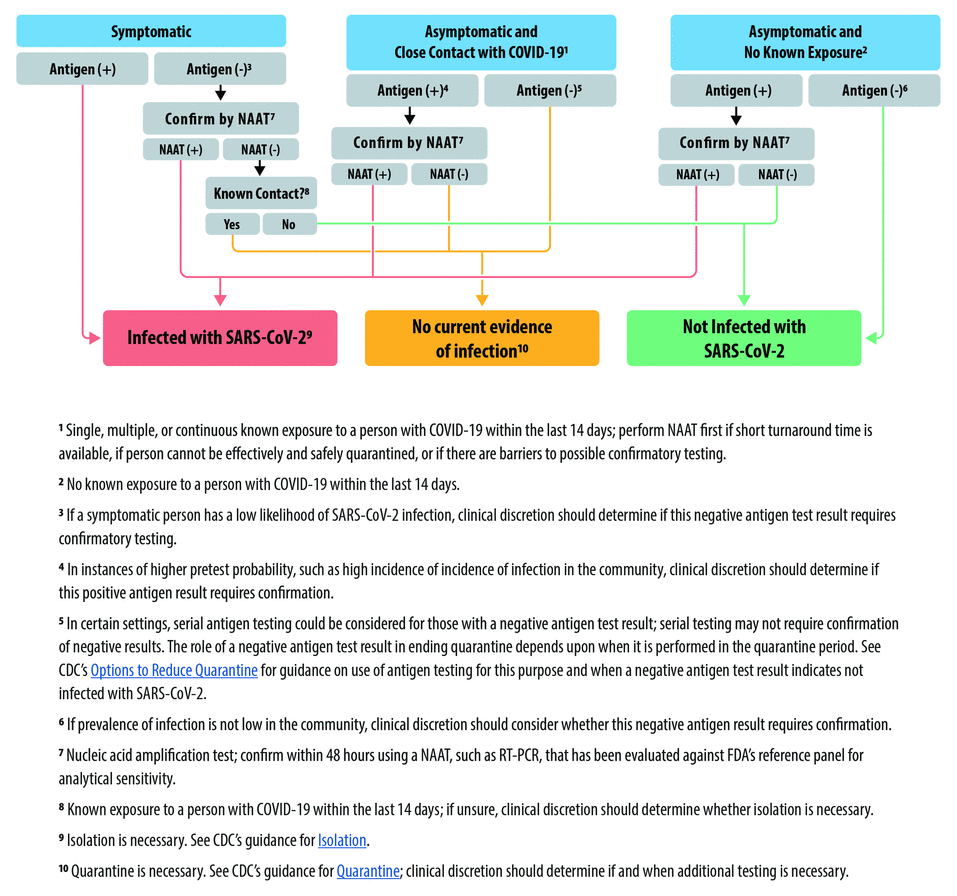
Studies have shown that isothermal amplification assays and antigen detection tests are not as sensitive as RT-PCR assays.10 This has implications for relying on negative results from these tests. Therefore, if a home COVID-19 test returns a negative result in a symptomatic individual, the result should be questioned, and that individual should not be considered free of SARS-CoV-2 infection. This is consistent with guidance from the CDC, as shown in Figure 1.7 If these individuals (ie, symptomatic but tested negative), who may very well have COVID-19, consider themselves uninfected, they may spread the infection to others. Retesting, in this situation, should be considered using a highly sensitive molecular assay. False-negative test results have been reported to the FDA for both antigen detection tests and isothermal amplification tests.
Centers for Disease Control guidance for the interpretation and follow-up for antigen detection tests could be generalized for any home COVID-19 test. From reference 7.
TESTING ACCURACY IN ASYMPTOMATIC INDIVIDUALS
Testing asymptomatic individuals (ie, those without the signs and symptoms of COVID-19) is uniquely challenging. These individuals may be uninfected, infected but asymptomatic, or presymptomatic (ie, will soon become symptomatic). Infected individuals may be very early in the course of disease with a low viral load or may have more established viral replication with viral loads similar to patients with symptomatic disease.16 The possibility of misclassifying an infected individual as negative will likely be exacerbated by the home COVID-19 tests because these tests are not as sensitive as the laboratory-based RT-PCR tests. Therefore, it is crucial that individuals, asymptomatic or otherwise, do not use a negative test to consider themselves infection free and relax mitigation strategies, such as masking and recommended social distancing.
Another significant hazard with the use of home COVID-19 testing (or any testing) in asymptomatic individuals is the increased likelihood of false-positive reactions. It should be noted that we selected the word “likelihood” rather than “possibility” as false-positive test results will occur in this setting. For example, the FDA emergency use authorization submission for the Ellume COVID-19 Home Test for asymptomatic individuals reported 15 positive results, 5 of which (33%) were false-positives!5
As noted before, this occurs largely because the pretest likelihood of disease in asymptomatic individuals is low (they do not have signs and symptoms of disease). The pretest likelihood of disease is increased if they have had close contact with a SARS-CoV-2– infected patient, which is reflected in the CDC algorithm for the interpretation of antigen detection tests (Figure 1). Therefore, when an asymptomatic individual tests positive on a home COVID-19 test, the possibility of a false-positive should be considered and the result confirmed using an alternative assay (ie, orthogonally confirmed).
It is worth noting, however, that a negative antigen test result is more reliable (ie, has a high negative-predictive value) for an asymptomatic individual with have no recent contact with a known case. Nevertheless, a negative result should not be used for making decisions concerning infection prevention. Regardless of the test results, individuals must adhere to the recommended infection prevention controls.
SUMMARY
Home COVID-19 testing increases the number of tests available, does not require a healthcare visit for specimen collection and testing, and provides quick results.
Home COVID-19 tests provide accurate positive results in symptomatic individuals.
Positive results in asymptomatic individuals are less accurate and should be confirmed by more accurate tests.
Negative test results, regardless of symptomology, do not obviate the need for ongoing mitigation strategies (eg, masking, social distancing, etc).
CONCLUSION
The FDA emergency use authorization of several home COVID-19 tests represents a significant advance in the tools available to bring the COVID-19 pandemic under control. These tests increase the overall number of tests available, and they enable individuals to test themselves in their homes and, importantly, to receive almost immediate results. These features allow infected individuals to remain in isolation, and they preserve personal protective equipment because healthcare providers are not needed to obtain the specimen for testing. Insurance coverage for home tests vary depending on the reason for testing (eg, illness versus travel), and it is unclear if tests that are available without a prescription will be covered. Individuals should check with their insurance providers for coverage details.
These home COVID-19 tests provide very accurate positive results in symptomatic individuals, based on the limited data submitted to the FDA. Negative test results in symptomatic individuals should be questioned because these tests are usually less accurate than the highly sensitive molecular tests performed in the laboratory. Similarly, positive results in asymptomatic individuals should be confirmed using an alternative assay. Finally, negative test results, regardless of symptomatology, do not mean that infection-mitigation strategies can be relaxed.
DISCLOSURES
Dr. Gary W. Procop has disclosed a financial relationship (research grant support) with Lucira Clinical Trial. Dr. Daniel D. Rhoads has disclosed financial relationships (research grant support or consulting fees) with Becton, Dickinson and company, BioFire Diagnostics, Bio-Rad Laboratories, Cepheid, Cleveland Diagnostics, Hologic, Luminex, OpGen, Talis Clinical, Centerline Biomedical, and Qiagen. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Footnotes
The statements and opinions expressed in COVID-19 Curbside Consults are based on experience and the available literature as of the date posted. While we try to regularly update this content, any offered recommendations cannot be substituted for the clinical judgment of clinicians caring for individual patients.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.