ABSTRACT
Coronary revascularization has matured as a field since coronary artery bypass grafting (CABG) was first developed over 50 years ago, with diagnostic and treatment methods having advanced dramatically. CABG remains the standard of care for obstructive coronary artery disease, particularly for patients with multivessel disease or diabetes. It is now recognized that not all CABG is created equal—operative strategy, including conduit choice for bypass grafts and target coronary selection, affects survival. A multidisciplinary approach including surgeons with a special interest in CABG is recommended to optimize treatment selection and outcomes.
The main criteria guiding the selection of revascularization therapy are disease stability, procedural risk, patient comorbidities, atherosclerotic burden, and lesion complexity.
In general, CABG is preferred over percutaneous coronary intervention in patients with a heavy atherosclerotic burden and diabetes, and those without multiple significant baseline comorbidities, frailty, or short life expectancy.
CABG with arterial grafts can improve patient longevity, particularly with appropriate patient and coronary artery target selection.
Multiple arterial grafts should be considered over single thoracic artery and multiple vein conduits.
Less-invasive strategies are emerging.
Guideline-directed medical therapy in coronary artery disease is essential for improved outcomes in primary and secondary prevention.
Coronary artery bypass grafting (CABG) has been performed for more than 50 years. And even though the procedure is increasingly being used for older and higher-risk patients, outcomes have improved substantially over time. The surgery has developed beyond a “cookie-cutter” generic cardiac operation, and the use of a multidisciplinary, experienced heart team approach has become important.
This review briefly describes:
The evolution of CABG
Guidance for diagnosing coronary artery disease and determining the best strategy for intervention
Conduit selection for CABG, including evidence supporting multiple arterial grafting
The emergence of less-invasive strategies
Enhanced recovery after surgery protocols
The importance of medications.
NEED FOR CABG IS GREAT
Every year, about 18 million Americans are diagnosed with coronary artery disease, the most common cause of death in the United States.1 The estimated annual incidence of new myocardial infarctions is 720,000, in addition to about 335,000 recurrent infarctions.1 Isolated CABG is the most common cardiac surgical procedure in North America.2
EVOLUTION OF A SURGERY
In 1968, Cleveland Clinic established CABG as the standard of care for obstructive coronary artery disease.3 Two years later, a Cleveland Clinic team led by René Favaloro4 reported on the workup and favorable outcomes of more than 300 patients who underwent “venous autograft reconstruction” with appropriate follow-up.4
All-venous-conduit CABG reigned from 1968 until January 1986, when Loop et al5 demonstrated improved graft patency and a 10-year actuarial survival with internal thoracic artery (ITA) grafts compared with saphenous venous grafts anastomosed to the left anterior descending (LAD) coronary artery (86.6% vs 75.9% survival). The authors acknowledged that a randomized controlled trial would be beneficial to confirm their findings, but that this would not be possible because “present knowledge about late patency rates would bias the offering of the internal mammary [thoracic] artery and saphenous vein as comparable conduits in a trial.”5 And they were right.
Pursuit of improved outcomes has intensified in the current era of public reporting. Perioperative mortality rates have been reported nationally at 2% (and at < 1% at some centers of excellence).3 But beyond perioperative mortality and morbidity, interest in improving long-term outcomes has grown. Debate continues about the use of bilateral ITA grafting and other multiarterial grafting strategies. Minimally invasive options and robotic assistance are also evolving.6 Given all these highly technical approaches requiring high-volume surgeon experience, some have recently called for coronary revascularization to be recognized as a subspecialty within cardiac surgery.7,8
DIAGNOSTIC METHODS HAVE ADVANCED
Coronary angiography remains the gold standard for diagnosing coronary artery disease.9 Optical coherence tomography, intravascular ultrasonography, fractional flow reserve, cardiac computed tomographic angiography, and cardiac magnetic resonance imaging (MRI) are newer diagnostic methods that provide more than a simple subjective visual estimation of coronary narrowing; they provide information on granular anatomic and physiologic features of coronary lesions and the downstream effect on the myocardium.
Role of fractional flow reserve
Stenosis seen by 2-dimensional angiography does not always reflect a flow-limiting lesion.10 In fact, residual stenosis determined by coronary angiography does not affect outcomes if the patient is completely revascularized by fractional flow reserve criteria.
In the setting of percutaneous coronary intervention (PCI) in multivessel coronary artery disease, fractional flow reserve has been found to be superior to coronary angiography. Unfortunately, this has not been rigorously studied for surgical revascularization.10,11 Extrapolating the utility of fractional flow reserve to CABG entails the risk of erroneously downgrading a multivessel disease scenario or underestimating disease severity and forgoing CABG for a less invasive but also less durable therapy.
We have only limited data to correlate fractional flow reserve with graft patency. While venous grafts are not vulnerable to competitive flow from native coronary vessels, arterial grafts are at risk for failure when bypassing less-than-severe lesions. Compared with radial grafts, ITAs appear to be less vulnerable to competitive flow, with no clear stenosis cutoff and with excellent long-term patency rates even when used to bypass moderately diseased vessels.12 Radial grafts should only be used to bypass occluded or severely diseased vessels.13
Cardiac MRI has evolved dramatically
Late gadolinium enhancement cardiac MRI is a noninvasive nonstress test that has become the most sensitive and specific viability test. Image resolution is superior to that of single-photon-emission computed tomography, and it identifies smaller, more distinct areas of fibrosis. Acutely, late gadolinium enhancement cardiac MRI can overestimate infarcts early due to tissue edema, but a transmural uptake of less than 50% infers functional improvement.
TREATMENT CONSIDERATIONS
Three main factors should be considered when deciding on an intervention strategy.
Disease stability. Stability of coronary artery disease and presentation—ie, ST-elevation myocardial infarction (STEMI), non-STEMI, or stable angina—are factored into the management algorithm. PCI is the treatment of choice for STEMI; for non-STEMI and stable angina, recommendations are more nuanced. In patients with stable coronary artery disease and low-risk anatomic features, PCI has failed to show convincing evidence of benefit beyond a modest reduction in angina.14,15 Comparisons of CABG and medical therapy are dated, and emphasis now is on complementary rather than competing therapies.16,17 Medical treatments (eg, high-intensity statins, dual antiplatelet therapy, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and novel glucose-lowering agents) are transforming primary and secondary cardiovascular prevention in patients with stable angina, resulting in reduced event rates in recent years.18 Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for patients with type 2 diabetes mellitus and renal impairment are associated with reduced disease progression and recurrent ischemic events.19
Procedural risk and patient comorbidities. CABG risk is most commonly and reliably estimated by the Society of Thoracic Surgeons risk calculator, which estimates the risk of perioperative mortality and major morbidity.20 The latter includes stroke, with about a 1% perioperative rate, which is slightly higher than the risk associated with PCI.21 Advanced age is an important risk factor for stroke and periprocedural mortality, but it should be considered in the context of other risk factors when choosing between therapies.
Risk models perform well at a population level but are limited for estimating risk for individuals, particularly for patients with rare comorbidities (eg, cirrhosis) or unique risk profiles. Patients with significant baseline comorbidities, frailty (not captured by the Society of Thoracic Surgeons calculator), and reduced life expectancy are best suited for PCI.
Atherosclerotic burden and disease complexity. Coronary artery disease complexity is often assessed using the Synergy Between PCI With TAXUS and Cardiac Surgery (SYNTAX) trial score,22 which is incorporated in the American College of Cardiology–American Heart Association criteria for treatment selection. A heavy atherosclerotic burden favors CABG over PCI.23
LEFT MAIN DISEASE
Historically, the mortality rate in untreated left main coronary artery disease is about 50% at 3 years.24 It is a heterogeneous condition that may involve the ostia, midshaft, bifurcation, or trifurcation. The specific areas involved affect the feasibility and success of PCI but have no bearing on CABG success or durability. The role of PCI vs CABG in left main disease is controversial, with 2 recent trials showing seemingly different findings. However, neither favored PCI over CABG.16
The 5-year Evaluation of XIENCE vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial showed noninferiority of PCI and CABG for left main disease, but an increased rate of all-cause mortality with PCI at 5 years.25
The 5-year Nordic-Baltic-British Left Main Revascularization (NOBLE) trial, while not powered for mortality, showed that PCI was inferior to CABG for left main disease for reintervention and nonprocedural myocardial infarction, a marker of mortality.26
About 10% of STEMIs involve the left main coronary artery. In STEMI or hemodynamic instability, PCI is the treatment of choice. In non-STEMI and stable ischemia, the American College of Cardiology–American Heart Association guidelines give the highest recommendation for CABG for all SYNTAX levels (class I, level of evidence A)27; PCI is recommended at this level only for low-risk SYNTAX scores.
MULTIVESSEL DISEASE
Left main and multivessel coronary artery disease are treated as different entities in the literature, even though less than 15% of lesions are isolated left main disease. SYNTAX 10-year data show an all-cause mortality benefit for CABG over PCI in patients with 3-vessel disease (21% vs 28%).28
Current guidelines recommend CABG over PCI for multivessel coronary artery disease in patients with diabetes and for those with left ventricular dysfunction.27 Even for severe left ventricular dysfunction (ejection fraction < 35%), CABG is associated with improved long-term outcomes, including survival, compared with PCI for patients with indications for CABG and who can tolerate the stress of surgery.29
Why CABG improves outcomes for left main and multivessel coronary artery disease is likely multifactorial. The distal insertion of a bypass graft is downstream from where most future atherosclerotic disease might develop. In addition, use of arterial grafts that are resistant to atherosclerosis enhances long-term patency. Data suggest that the incremental benefit of CABG is strongly associated with the use of the ITA.30 Finally, surgical revascularization more frequently achieves complete revascularization, which is associated with improved survival.
CONDUIT SELECTION FOR CABG
Conduit selection is a current topic of debate.
Saphenous vein. Attrition of the saphenous vein graft, the Achilles’ heel of CABG, occurs in phases. The first phase is nearly immediate and likely related to a technical factor. This can be avoided with intraoperative evaluation of the bypass graft. Transit-time flow meters can identify low graft flows due to thrombosis, kinking, conduit dissection, coronary dissection, or anastomosis stenosis, all of which are potentially correctable.31 Subsequent phases of vein graft failure include intimal hyperplasia and atherosclerosis. Saphenous vein graft attrition rates of 1% to 2% per year for the first 6 years and 4% per year for the next decade have been reported.32
Arteries vs veins. Dimitrova et al33 reported that angiography over a 15-year period revealed that coronary territories bypassed with arteries had less disease progression compared with territories bypassed with veins. The internal elastic lamina of arterial grafts protects them from disease progression. Native coronary disease is also protected by arterial grafts for unclear reasons, but possibly due to the downstream effect of vasoactive signals.34
ITA and radial artery grafts. At 15 years, right ITA graft patency is reported to be more than 90% and left ITA graft patency more than 95%.35 The Society of Thoracic Surgeons guidelines13 recommend the following:
ITA grafts should be used to bypass the LAD artery when bypass of the LAD artery is indicated (class of recommendation [COR] I, level of evidence [LOE] B)
As an adjunct to a left ITA graft, a second arterial graft (right ITA or radial artery) should be considered in appropriate patients (COR IIa, LOE B)
Use of arterial grafts (including specific targets, number, and type) should be a part of the discussion of the heart team in determining the optimal approach for each patient (COR I, LOE C).
In 2019, RADIAL study 5-year data showed a benefit for using the radial artery rather than the saphenous vein for graft occlusion and target revascularization.36 Rates of myocardial infarction and repeat revascularization were also superior for radial arteries, and a mortality benefit was reported in a follow-up study.37
SINGLE VS MULTIPLE ARTERIAL GRAFTING
Evidence favors multiarterial options
In 2019, the Arterial Revascularization Trial (ART) 10-year intention-to-treat data showed no difference in survival or event-free survival for bilateral vs left ITA. However, a 14% crossover rate, excellent medical compliance, and a radial artery conduit in more than 20% of patients possibly clouded the results.38 A post hoc as-treated analysis showed improved mortality and major adverse cardiac and cerebrovascular events with multiple arterial grafting. Additionally, a 5-year post hoc analysis found that radial artery grafting improved outcomes in both groups.39
Since 2001, 5 major systematic reviews and 1 meta-analysis found that bilateral ITA grafting offered a survival advantage over left ITA grafting, including long-term survival, reduced hospital mortality, reduced cerebrovascular accidents, and reduced revascularization.38
Despite evidence of the benefits of multiple arterial grafting and the professional association recommendations to encourage its use, only a small percentage of patients undergoing CABG in the United States receive multiarterial grafts. Reasons for this include additional technical complexity, prolonged operative times, and potential for complications.40
Regional practice differences
In California, receipt of a second arterial graft decreased from 10.7% of isolated CABG operations in 2006 to 9.1% in 2011, with the use of a radial artery graft falling from 7.8% to 6.6% and a right ITA graft from 3.0% to 2.4%.41 Despite these trends, there is a clear survival advantage for multiarterial grafting 7 years after surgery.
Chikwe et al42 performed a retrospective cohort analysis with propensity matching. Of patients undergoing CABG between 2005 and 2012, 14% received multiarterial grafting, a nearly 50% higher rate than was found in the California study. Patients receiving multiarterial grafts were younger and healthier at baseline. After propensity matching, those receiving multiarterial grafts had better 10-year survival and lower 10-year myocardial infarction and reintervention rates. However, the study also identified subgroups of patients, including those with advanced age or renal disease, who might not realize additional benefits from multiarterial grafting.
Ongoing trial may provide standard
The ongoing Randomization of Single vs Multiple Arterial Grafts (ROMA) trial is expected to be the definitive prospective randomized trial comparing multiple arterial grafting vs a single ITA to the LAD artery with saphenous vein graft bypasses to the remaining targets.43 The enrollment goal is 4,300 patients, and the composite outcomes include death, stroke, myocardial infarction, and repeat revascularization.
Optimizing success of multiarterial grafts
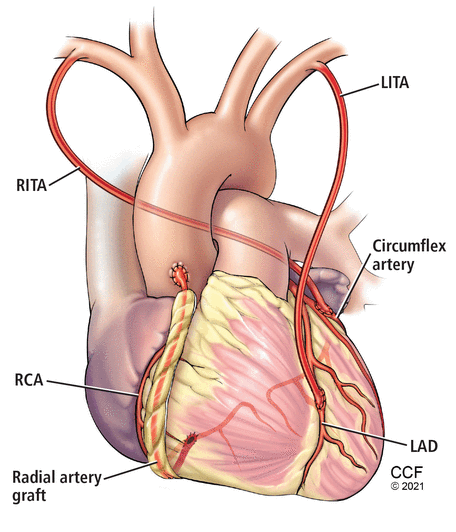
Multiple arterial grafting (Figure 1) is not without its nuances, including conduit choice and intended target coronary vessel. For example, radial artery grafts are best used to bypass severely diseased target vessels to minimize competitive flow and optimize graft patency.13 The myocardial mass supplied by a diseased vessel is also critically important. Important target vessels extend more than 75% of the way to the apex of the heart. Matching important vessels (extending more than 75% to the apex) with the second arterial graft has a long-term mortality benefit.44
An example of multiarterial coronary artery bypass grafting. The left internal thoracic artery (LITA) is used to bypass the left anterior descending artery (LAD), the right internal thoracic artery (RITA) to bypass the circumflex artery, and the radial artery to bypass the right coronary artery (RCA).
The feared risk of sternal wound complications associated with bilateral ITA harvesting can be mitigated by meticulous harvesting techniques and ITA skeletonization.45 Skeletonization separates the ITA from adjacent tissues, with the surgeon staying close to the ITA wall throughout the dissection, thereby reducing adjacent tissue damage and preserving collateral routes of blood flow to the sternum compared with techniques that take the ITA as a pedicle that incorporates adjacent chest wall tissues. There is a theoretical risk of increased ITA injury in the hands of inexperienced harvesters, but data on the differential patency rates between skeletonized vs pedicled ITAs are limited.
The importance of an experienced coronary surgeon in decision-making and the performance of CABG cannot be overstated.7,8 A specific volume-outcome relationship has been described for bilateral ITA grafting.46 The increased risk associated with surgery for complex revascularization procedures such as redo CABG is well documented47 but is mitigated by surgical expertise.48 In addition, a focused interest in CABG facilitates innovation and the development of less invasive approaches.
LESS-INVASIVE CABG STRATEGIES
Off-pump CABG avoids use of cardiopulmonary bypass and is physiologically less invasive than traditional on-pump CABG. Off-pump CABG can benefit select high-risk patients not typically enrolled in trials. Surgical experience is critical in mitigating reduced graft patency and incomplete revascularization associated with off-pump CABG.49 Widespread adoption is ill-advised, and indeed, use of off-pump CABG has declined.
Robotic CABG accounts for less than 1% of CABG operations in the United States.6 Data supporting use of these procedures outside of select specialized centers are currently limited. Technology is lagging, and it is difficult to teach robotic multiarterial CABG and reliably achieve complete revascularization.
Hybrid CABG uses robotic or minimally invasive left ITA harvest with a direct hand-sewn left ITA-to-LAD artery anastomosis through a minithoracotomy (Figure 2). Non-LAD artery stenosis is then addressed with drug-eluting stents. Theoretical benefits are lower occurrence of stroke, decreased infection, sternal sparing, fewer transfusions, and faster recovery. The Safety and Efficacy of Hybrid Revascularization in Multivessel Coronary Artery Disease study (POL-MIDES) found no difference between traditional and hybrid CABG in outcomes at 1 and 5 years.6 Other trials are ongoing, and more are expected in the future.
Example of minimally invasive coronary artery bypass grafting, performed through a small left thoracotomy incision, in which the left internal thoracic artery is bypassed to the left anterior descending artery without use of a heart-lung machine. The patient’s head is toward the top, and the skin marking is where a traditional sternotomy incision is placed.
OPTIMIZING RECOVERY AFTER SURGERY
Enhanced recovery after surgery relies on evidence-based protocols designed to improve outcomes and cost-savings based on rigorous data review and protocol development.50 Postoperative goal-directed hemodynamic resuscitation algorithms reduce 30-day major adverse cardiovascular events in high-risk patients.51 Similarly, fast-track early extubation protocols decrease time on a ventilator. Shorter extubation times are associated with decreased length of stay and hospital cost.52
Opioid-sparing pain management
In this era of opioid abuse, pain management has come under global public scrutiny. More importantly, opioid-sparing techniques are improving patient outcomes and decreasing length of stay. Minimizing opioid use also reduces the incidence of delirium. Some form of delirium can occur in nearly 50% of postoperative cardiac surgery patients, increasing hospital mortality and readmission and decreasing long-term survival.50 Many causes of delirium are reversible, and frequent delirium screening by bedside nurses and critical care teams improves outcomes.
Glycemic control
Multiple mechanisms to deal with postoperative complications secondary to hyperglycemia exist. Goal blood glucose levels of 80 to 110 mg/dL are well established.53 Glucose levels over 160 to 180 mg/dL managed with insulin infusions have improved outcomes, including reduced infections.
SECONDARY PREVENTION
Optimal medical management for secondary prevention and improved long-term outcomes after CABG has been increasingly recognized.54 Discharge prescriptions for beta blockers and statins are process measures tracked by the Society of Thoracic Surgeons as part of its program quality ratings. The benefits of beta blockers include a potential decrease in long-term mortality after CABG.55 In patients receiving radial artery grafting, use of antispasmodic medications, including calcium channel blockers, is associated with improved outcomes.56 Statin use after surgery is associated with decreased readmissions and late death from myocardial infarction or stroke.57
Dual antiplatelet therapy is now recommended for 6 months in patients with acute coronary syndrome undergoing CABG. Additionally, in patients who had coronary stenting prior to CABG, dual antiplatelet therapy may prolong stent patency and prevent thrombus development and propagation.58
Comprehensive rehabilitation programs have been developed to prevent readmissions and improve treatment compliance and quality of life after discharge. Medication adherence dramatically improves outcomes regardless of coronary revascularization strategy.59 For patients who do not adhere to medications, CABG leads to improved major cardiac event-free survival. New methods of improving treatment adherence are currently being evaluated; they include wearable technology, educational tools, and increased use of virtual visits.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.