A 65-year-old man with a history of hypercholesterolemia and hypertension well controlled on losartan 25 mg daily presents for follow-up on his cholesterol. He has no history of smoking, alcohol use, or heart disease. In addition to losartan, he has been taking rosuvastatin 40 mg daily for the past 2 months. Despite these measures, he has been unable to achieve his goal low-density lipoprotein cholesterol (LDL-C) level of less than 100 mg/dL. His lipid panel is LDL-C 165 mg/dL, high-density lipoprotein 45 mg/dL, and total cholesterol 210 mg/dL. Before starting statin therapy, his lipid panel was LDL-C 185 mg/dL, high-density lipoprotein 45 mg/dL, and total cholesterol 230 mg/dL. His current 10-year risk of atherosclerotic cardiovascular disease (ASCVD) is 14.6%. What are the next steps in managing this patient’s hypercholesterolemia?
In adults at risk of ASCVD, multiple factors can account for lack of response to statin therapy, ranging from poor compliance to other diagnoses. Further diagnostic studies may be indicated and other treatments can be considered if LDL-C goals are not met after a trial with statin therapy.
STATIN HYPORESPONSIVENESS DEFINED
Statin hyporesponsiveness is the inability to achieve target LDL-C levels despite maximally tolerated more potent statin therapy.1 Target LDL-C varies based on ASCVD risk; according to the latest American College of Cardiology guidelines, the target includes a percent reduction and a goal level.2
For primary prevention, it is recommended that patients age 40 to 75 with intermediate ASCVD risk (7.5% to < 20%) achieve a 30% to 49% reduction in LDL-C with a goal LDL-C of less than 100 mg/dL.2,3 The recommendation for patients with high ASCVD risk (≥ 20%) is a 50% or greater reduction in LDL-C with a goal LDL-C of less than 70 mg/dL.2,3
For secondary prevention in patients age 40 to 75 with ASCVD labeled not very high risk, the recommendation is also LDL-C reduction of 50% or greater and a goal LDL-C of less than 70 mg/dL.2,3 For secondary prevention in very-high-risk patients, including those who have a history of either multiple major ASCVD events or 1 major ASCVD event with multiple high-risk factors, the goal is LDL-C reduction of 50% or greater and a lower goal LDL-C of 55 mg/dL.2–4
Inability to achieve these targets on statins alone is deemed an insufficient response to statins.
EVALUATING STATIN HYPORESPONSIVENESS
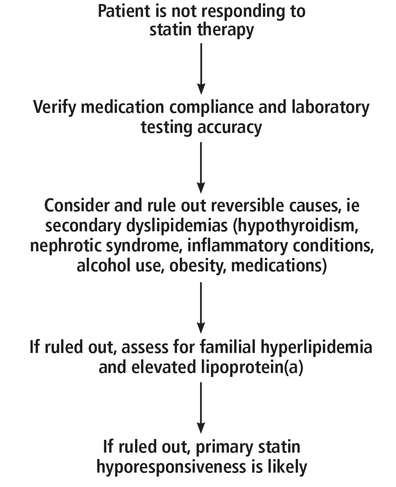
Factors contributing to statin hyporesponsiveness can be multifactorial and include medication nonadherence, underlying lipid disorders, pharmacogenomic factors, and environmental factors.1 Evaluation of statin resistance requires a comprehensive review of all potential causes (Figure 1).5
Clinical approach to evaluating statin hyporesponsiveness.
Based on information from reference 5.
Noncompliance and analytic error in laboratory testing
The first steps are to ensure that patients are taking their medication and that laboratory testing is accurate. Statin noncompliance is the most commonly cited reason for persistent hypercholesterolemia.6 Factors contributing to noncompliance include pill burden and, in some cases, side effects such as myalgias. Patients should be asked routinely about their statin use, and particularly about when they take their statin, as some statins are most effective when taken at bedtime. Some clinicians monitor compliance with electronic health records, patient questionnaires, and routine pill counts.5
Patients whose noncompliance is related to statin intolerance due to side effects such as myalgias may respond to an alternative statin, a lower-dose statin, intermittent dosing, or an alternative lipid-lowering agent.7 Typically, at least 2 different statins should be tried before transitioning to an alternative lipid-lowering agent.5 Notably, a large meta-analysis that included more than 4 million patients showed an overall prevalence of statin intolerance of 9.1%, suggesting that the prevalence of statin intolerance may be overestimated.8
Laboratory test inaccuracy due to LDL-C variations in fasting vs nonfasting states can make a patient appear to be statin-hyporesponsive. It is essential to repeat testing on multiple occasions and note the fasting state so that LDL-C values can be compared over time. Other methods of calculating LDL-C that are less affected by triglyceride levels, such as the Sampson-NIH or Martin-Hopkins equations, can also be used to ensure accuracy.5
When compliance and laboratory test accuracy have been addressed, secondary dyslipidemia, common lipid disorders such as familial hypercholesterolemia, and elevated lipoprotein(a) should be considered.
Secondary dyslipidemias
The workup for statin-hyporesponsive hypercholesterolemia begins with ruling out reversible causes of hypercholesterolemia, or secondary dyslipidemias. These include hypothyroidism, nephrotic syndrome, inflammatory conditions, alcohol use, obesity, and medications. Common medications that can cause hyperlipidemia include antiretroviral therapy for human immunodeficiency virus infection, amiodarone, phenytoin, carbamazepine, corticosteroids, and cyclosporine. When a reversible cause of secondary dyslipidemia is identified, the first step is treatment of the underlying cause followed by repeat LDL-C testing. If the LDL-C is still elevated, a second lipid-lowering agent can be added.5
Familial hyperlipidemia
If secondary dyslipidemia is ruled out, the evaluation should assess for familial hypercholesterolemia caused by mutations in the gene encoding the LDL receptor (LDLR).5 The Dutch Lipid Clinic Network criteria,9 Simon Broome criteria,10 or the American College of Cardiology/American Heart Association guidelines can be used for diagnosis.11 The major forms of familial hypercholesterolemia are heterozygous and homozygous5:
Heterozygous familial hypercholesterolemia consists of mutations in 1 allele or different mutations in both alleles, and LDL-C levels can be 2 to 3 times above normal
Homozygous familial hypercholesterolemia consists of the same mutation in both alleles, and LDL-C can be up to 10 times above normal.
Response to statin therapy in familial hypercholesterolemia depends on the remaining function of the LDL receptor, which is determined by the type of mutation present. Patients with LDLR mutations that completely inactivate receptor activity are often resistant to statins altogether. Some patients with familial hypercholesterolemia may benefit from a second lipid-lowering agent in addition to statin therapy, but many patients, particularly those with the homozygous form, do not benefit from second agents and ultimately require referral to a lipid specialist.5
Elevated lipoprotein(a)
The workup should include measurement of lipoprotein(a), an LDL-like molecule with a prothrombotic apolipoprotein(a) protein attached to the atherogenic apolipoprotein B-100 component.5,12 The combination of the atherogenic apolipoprotein B-100 component with a prothrombotic apolipoprotein(a) results in markedly increased ASCVD risk that is not reduced by lifestyle changes, statins, or other lipid-lowering agents.12 Traditional LDL-C calculations reported on lipid panels include lipoprotein(a), and it is reasonable to check the lipoprotein(a) level when assessing for statin hyporesponsiveness. If it is elevated, an additional nonstatin agent could be added to maximize LDL-C lowering.5
No treatments targeting lipoprotein(a) specifically are approved, but trials are under way.12 Examples include antisense oligonucleotides like pelacarsen that bind apolipoprotein(a) messenger RNA to prevent translation; small interfering RNA molecules like olpasiran and lepodisiran that degrade apolipoprotein(a) messenger RNA; and oral agents such as muvalaplin that disrupt the noncovalent interactions between apolipoprotein(a) and apolipoprotein B-100.12
PRIMARY HYPORESPONSIVENESS
If the initial workup is negative, then primary statin hyporesponsiveness can be considered. Pharmacogenetic factors likely drive primary statin hyporesponsiveness. Genetic mutations affecting statin responsiveness can be involved in either the lipid metabolic pathway or metabolism of the drug itself. Commonly affected genes (and the proteins they encode) in the lipid metabolic pathway include APOA1 (apolipoprotein A1), LPA (apolipoprotein[a]), and PCSK9 (proprotein convertase subtilisin/kexin type 9); genes involved in drug metabolism that are affected include SLCO1B1 (organic anion transporting polypeptide 1B1), CYP3A4 (cytochrome P450 3A4), and CYP7A1 (cytochrome P450 7A1). Although pharmacogenetic testing can be pursued, it may have low clinical significance, and it would be reasonable to instead add a second nonstatin agent.5
NONSTATIN ALTERNATIVES
Statins remain the primary treatment for patients with hypercholesterolemia, but newer nonstatin cholesterol-lowering agents can be used for patients with statin-resistant hypercholesterolemia (Table 1).2,3,13 Ezetimibe, a first-line nonstatin therapy, inhibits cholesterol absorption in the small intestine and reduces LDL-C levels up to 25% when taken in combination with a statin.3,13,14
Nonstatin lipid-lowering agents
PCSK9 inhibitors such as evolocumab and alirocumab are also effective. These are monoclonal antibodies that bind PCSK9 molecules and subsequently prevent LDL receptor degradation. This class of lipid-lowering agents has been shown to reduce LDL-C levels by 55% to 65% when added to statin therapy.13 Inclisiran, a small interfering RNA molecule, is an effective LDL-C–lowering agent that also acts on PCSK9 and catalyzes the breakdown of PCSK9 messenger RNA.
Bempedoic acid is an adenosine triphosphate citrate lyase inhibitor that lowers LDL-C by inhibiting cholesterol synthesis upstream of statins. Evinacumab is an angiopoietin-like 3 inhibitor that drives increased lipid metabolism, and lomitapide inhibits apolipoprotein-B assembly, leading to reduced LDL-C levels.3
Selecting a nonstatin
Initial treatment for all patients at risk of ASCVD should include statin therapy to achieve LDL-C targets as outlined by the American College of Cardiology expert consensus decision pathway for nonstatin therapies.2 Additional agents can be considered for patients unable to achieve their target LDL-C despite maximally tolerated statin therapy. The initial nonstatin agent of choice is ezetimibe because of its cost, safety profile, and tolerability.2,3 If LDL-C targets are not met with ezetimibe, then PCSK9 inhibitors can be used in addition to or in place of ezetimibe.
If a patient requires a greater than 25% reduction in LDL-C despite treatment with maximally tolerated statin therapy or is deemed to be very high risk (eg, an LDL-C greater than 190 mg/dL), it is reasonable to initiate PCSK9 inhibitors before trying ezetimibe; ezetimibe typically can only lower LDL-C by 25%.3,14 Inclisiran or bempedoic acid can also be used in these very-high-risk patients.
Patients with homozygous familial hypercholesterolemia benefit the most from agents such as evinacumab and lomitapide.3
THE BOTTOM LINE
Many patients do not meet their target LDL-C levels with statin therapy alone and require further investigation for causes such as secondary dyslipidemia, familial hypercholesterolemia, and elevated lipoprotein(a). The advent of novel, nonstatin lipid-lowering agents offers more options for lowering LDL-C levels. For patients who have an inadequate response to statin therapy, nonstatin lipid-lowering agents should be introduced alongside statin therapy to further reduce ASCVD risk, as recommended by the American College of Cardiology expert consensus decision pathway for nonstatin therapies.2
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.