ABSTRACT
To date, there are no effective antiviral medications for COVID-19. Drug repurposing, a strategy that uses existing drugs, offers potential prevention and treatment options for COVID-19. We discuss one treatment strategy that combines anti-inflammatory (melatonin) and antiviral (toremifene) agents for patients infected with SARS-CoV-2 from network medicine-based findings. We also describe the pathobiology and immunologic characteristics of COVID-19 and highlight the rationale of combination drug treatment to rescue the pulmonary and cardiovascular conditions resulting from COVID-19. A preliminary analysis reveals a high potential for the synergistic effects of melatonin and toremifene to reduce viral infection and replication, and the aberrant host inflammatory responses, offering strong biologic plausibility as an effective therapy for COVID-19.
PATHOBIOLOGY OF COVID-19
Human coronaviruses, a large family of viruses that has spread from animal hosts (eg, bats and civets) to humans, cause life-threatening respiratory diseases such as the Middle East respiratory syndrome (MERS-CoV) and severe acute respiratory syndrome (SARS-CoV-1). Coronavirus disease 2019 (COVID-19), caused by the virus SARS-CoV-2, has rapidly spread worldwide, affecting more people than SARS-CoV-1 and MERS combined. Morbidity and mortality from COVID-19 rises dramatically with age and coexisting disease comorbidities, with evidence also identifying race- and male-specific vulnerabilities.
Recent studies suggest that the immune system plays a key role in the decline and death of patients with COVID-19. Compared to mild disease, severe COVID-19 more frequently has elevated levels of cytokines, such as IL-2R, IL-6, IL-10, and TNF-α.1 Mechanistically, SARS-CoV-2 requires host cellular factors such as angiotensin-converting enzyme 2 (ACE2), to bind with the spike protein, and further causes viral replication and infectious process.2 ACE2 naturally protects against acute lung injury, which explains the increased lung pathophysiology and pathobiology (eg, acute respiratory distress syndrome [ARDS], pneumonia, and lung injury) due to dysregulation of ACE2 resulting from binding to the spike protein of SARS-CoV-2.2,3
NETWORK MEDICINE MEETS COVID-19 DRUG DISCOVERY
The emergence of COVID-19 has led to a rapid rise in case count and a mounting death toll, thereby substantiating an urgent need to identify therapeutics. Lack of effective antiviral treatment, rapid viral transmission rate, and a case-fatality rate of 2% to 6% collectively underscores the need for effective treatment. Drug repurposing could significantly shorten the time and reduce the cost compared with de novo drug discovery.4–7
SARS-CoV-2 requires host cellular factors (such as ACE2) for successful replication during infections.8,9 Systematic targeting of the virus-host protein-protein interactions (PPIs) offers a novel strategy for the development of effective drug repurposing (such as melatonin and toremifene) for COVID-19, as demonstrated in our recent study conducted here at Cleveland Clinic.10 We showed that a systems pharmacology-based network medicine platform, which quantifies the interplay between the virus-host interactome and drug targets in the human protein-protein interactome network, will offer clinically relevant repurposable drugs and drug combinations for effective treatment of COVID-19. Our hypothesis is based on the following: (1) the proteins that functionally associate with viral infection are localized in the corresponding subnetwork within the comprehensive human PPI network; and (2) the proteins that serve as drug targets for a specific disease may also be suitable drug targets for potential antiviral infection owing to common PPIs and functional pathways.
This methodology incorporates virus-host interactions from SARS-CoV-2, public drug-target databases, and the human protein-protein interactome. Specifically, we utilized a network proximity measure that quantifies the relationship between COVID-19 disease modules (formed by SARS-CoV-2 host genes/proteins) and drug targets in the human PPI network.10 The network proximity measure suggests that each drug-COVID-19 has a well-defined network-based footprint in the human interactome (ie, closer network proximity between targets of a drug and SARS-CoV-2 host genes/proteins indicate a biologically relevant relationship for potential treatment of COVID-19).
Using the network proximity measure, we successfully identified 16 highly repurposable drugs for COVID-19, which were further validated by enrichment analyses of drug-gene signatures and SARS-CoV-1–induced transcriptomics data in human cell lines.10 Using this approach, we were able to establish that a combinatorial drug treatment using melatonin and toremifene will provide an effective therapeutic strategy to mitigate the severity of COVID-19.
MELATONIN AS A CANDIDATE THERAPEUTIC IN COVID-19
Given the well-described lung injury characteristics and immune responses (cytokine storm) inherent to severe COVID-19, drugs that dampen the immune responses may offer effective treatment approaches for COVID-19 patients. Melatonin plays a key role in the regulation of our circadian rhythm, a homeostatic mechanism composed of a balance of interacting cellular circadian clocks and environmental influences that operate to inform our biologic clock and rhythmically alter the translation of thousands of genes, including melatonin-mediated anti-inflammatory and immune-related effects for COVID-19.11
Melatonin levels decline with age and, thus, exogenous melatonin administration may be of particular benefit to older patients. Melatonin suppresses NLRP3 inflammasome activation induced by activators such as cigarette smoking,12 and it attenuates pulmonary inflammation via reduction of nuclear factor-kappa B (NF-κB), transcription factor p65, and TNF-α13,14
Melatonin has shown antiviral effects by suppressing multiple inflammatory pathways that include IL6, IL1β, and TNFα.15 These inflammatory effects are directly relevant given the pulmonary and cardiovascular injury from severe COVID-19. Additional benefits of melatonin could include the regulation of the host circadian rhythms that could provide additional protective mechanisms to combat the SARS-CoV-2 infection.
SELECTIVE ESTROGEN-RECEPTOR MODULATORS AS A CANDIDATE THERAPEUTIC IN COVID-19
An overexpression of estrogen receptor plays a crucial role in blocking viral replication and infectious processes. Toremifene (Figure 1), a first-generation non-steroidal selective estrogen-receptor modulator, has shown antiviral activities against the Ebola virus,16,17 MERS-CoV,18 SARS-CoV-1,19 and SARS-CoV-220 in established virus-infected cell lines (Table 1). Compared with the classical estrogen receptor-related antiviral pathway, toremifene can prevent fusion by interacting with and destabilizing the virus membrane glycoprotein, and eventually blocking viral replication of the Ebola virus.16 The underlying antiviral mechanisms of SARS-CoV-1 and SARS-CoV-2 are unclear and should be a focus of future investigations.
A proposed model that combines antiviral (toremifene) and anti-inflammatory (melatonin) agents for effective treatment of COVID-19. Melatonin, a synthesized hormone, originated about 2.5 billion years ago and is evolutionarily conserved in all organisms from bacteria to humans. Melatonin exerts its antiviral activities by suppressing multiple inflammatory pathways, including IL6, IL1β, and TNFα, which are directly relevant given the lung injury characteristics of severe COVID-19. Toremifene, a selective estrogen receptor modulator FDA-approved to treat advanced breast cancer, has shown various antiviral activities against the Ebola virus, MRES-CoV, SARS-CoV-1, and SARS-CoV-2. Thus, the synergistic antiviral and anti-inflammatory effects of melatonin and toremifene offer a candidate treatment approach for COVID-19.
Literature-reported antiviral activities of toremifene
Toremifene is FDA-approved to treat advanced breast cancer in postmenopausal women, and it has been studied in men with prostate cancer (about 1,500 subjects) with reasonable tolerability.21 Toremifene is 99% bound to plasma protein with good bio-availability and is typically administered at a dosage of 60 mg orally.22 The combination of consistent findings from cell line data supporting antiviral effects of toremifene against the coronaviruses along with reasonable tolerability provide the basis to pursue it as a candidate therapy against SARS-CoV-2.
NETWORK-BASED DESIGN RATIONALE OF DRUG COMBINATION THERAPY FOR COVID-19
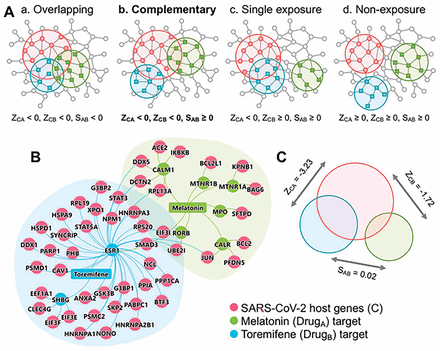
Drug combinations offering increased therapeutic efficacy and reduced toxicity play an important role in treating COVID-19; however, our ability to identify and validate effective combinations is limited by both the large number of drug pairs and dosage combinations. In our study, we proposed a novel network-based methodology to identify clinically efficacious drug combinations.23 Using FDA-approved drug combinations for hypertension and cancer, we found that a drug combination was therapeutically effective only if it was captured by the complementary exposure pattern: the targets of the drugs both hit the disease module but target separate neighborhoods (Figure 2A).
Network-based rational design of effective drug combinations for COVID-19. (A) Four possible exposure modes of the COVID-19 disease module to the targets of pairwise drug combinations. An effective drug combination can be captured by the complementary exposure pattern in which the targets of the drugs both hit the COVID-19 disease module (molecular determinants of disease pathophysiology in the human interactome) but target separate neighborhoods in the human protein-protein interactome network. In model, ZCA and ZCB denote the network proximity (Z-score) between drug targets (drugs A and B) and COVID-19 disease module. SAB denotes the separation score of drug targets. (B and C) The complementary exposure pattern of melatonin (a hormone synthesized in the pineal gland) plus toremifene (a selective estrogen receptor modulator) on the COVID-19 disease module, as described in our network medicine methodologies.10,23 For a network-based approach to COVID-19 drug combinations to be effective, we need to establish whether the topological relationship between two drug-target modules reflects biological and pharmacological relationships while also quantifying their network-based relationship to COVID-19 disease modules in the human protein-protein interactome.10
Our key methodology is that a drug combination is therapeutically effective only if it follows a specific relationship to the disease module, as captured by complementary exposure patterns in targets modules of both drugs (Figure 2A) without overlapping toxic mechanisms.23 For a pairwise drug combination, we used the network proximity (Z-score) measure to calculate the network relationship of targets for drug A (Zca) and drug B (Zca) with the COVID-19 disease module (Figure 2, B and C). We utilized a separation measure (Sab) to quantify target localization of two drugs’ targets in the human interactome. We defined a drug-drug-disease combination as complementary exposure pattern (Figure 2A): Two separated drug-target modules that overlap individually with the disease (eg, COVID-19 module). As we demonstrated in our previous study, a drug pair with a complementary exposure relationship to the disease module will have a higher potential for effective drug combination therapies.23
Using this methodology, we recently demonstrated that melatonin and toremifene will be an effective drug combination for COVID-19 (Figure 2, B and C). Given the up-regulation of systemic inflammation in patients with COVID-19 (eg, TNF-α, IL-6, IL-10, GCSF, MCP1)24,25—in some cases culminating in a cytokine storm observed in severe COVID-1926— combination therapy with an agent targeting inflammation (melatonin) and one with direct antiviral effects (toremifene) has high potential for treatment success. Yet, the optimal doses of melatonin plus toremifene warrant investigation in preclinical models. A challenge, however, is that SARS-CoV-2 replicates poorly in animals, including dogs, pigs, chickens, and ducks,27 which limits the utility of experimental animal models. Physiologically based pharmacokinetic modeling28–30 may be an ideal approach to determine the optimal dose of individual drugs and evaluation of adverse drug-drug interactions, such as QT prolongation, before clinical trials.
PERSPECTIVES AND FUTURE DIRECTIONS
As COVID-19 patients flood hospitals worldwide, there is an increased need for effective antiviral therapies to mitigate the effects of the viral infection. Multiple vaccine trials are under way; however, even if an effective vaccine is found, it will not be possible to generate a large number of vaccines in a short period to effectively combat the COVID-19 pandemic.
More than 400 drug trials are being conducted worldwide, based on ClinicalTrials.gov database (May 12, 2020). Remdesivir, an experimental viral RNA polymerase inhibitor originally developed for Ebola virus disease, was recently shown to improve oxygen-support status for COVID-19 patients.31 Yet, recent randomized controlled trials reported inconsistent clinical benefits with remdesivir. Beigel et al32 showed that remdesivir shortened the time to recovery in adults hospitalized with COVID-19 (ClinicalTrials.gov number, NCT04280705), while Goldman et al33 reported that remdesivir did not show a significant difference between a 5-day course and a 10-day course for patients with severe COVID-19 who do not require mechanical ventilation. Thus, clinical benefits of remdesivir are urgently needed to be investigated further in large-scale randomized controlled trials.
The urgent need for effective treatment led the FDA to issue an Emergency Use Authorization (EUA) for hydroxychloroquine to treat patients with COVID-19 despite the absence of evidenced-based clinical trials; however, various adverse effects (including QT prolongation) limit its clinical use in these patients, especially in those with pre-existing cardiovascular disease or diabetes.34–36 Among patients hospitalized in metropolitan New York with COVID-19, treatment with hydroxychloroquine was not significantly associated with differences in in-hospital mortality.37 On June 15, 2020, the FDA revoked this EUA for hydroxychloroquine citing concern for cardiac arrhythmia risk.38 A recent trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19 did not support significant benefit compared to standard care, further highlighting the challenges of identifying effective antiviral therapies.39
Development of effective treatments with low adverse events (eg, cardiotoxicity) is urgently needed for COVID-19 patients. Combinatorial drug identification using a network–based approach is an effective strategy to rapidly repurpose existing drugs for COVID-19 clinical trials that have the potential to provide an effective treatment strategy. Our drug repurposing data show promise for synergistic benefits of combination therapy with toremifene and melatonin, which appears to be driven by salutary anti-inflammatory, immunomodulatory, and direct antiviral mechanisms. A proof-of-concept of com bining remdesivir and anti-inflammatory drug baricitinib (an approved drug for treatment of adults with moderately to severely active rheumatoid arthritis) is under way to being tested in patients with COVID-19 (ClinicalTrials.gov Identifier: NCT04373044). This work highlights the ideal application of network-based drug repurposing strategies to expeditiously identify novel combination therapeutics, particularly in the setting of a medical crisis posed by the COVID-19 pandemic, and it sets the stage for rapid implementation of clinical trials to assess efficacy of these promising, candidate therapeutics.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under Award Number R00HL138272 and the National Institute of Aging under Award Number R01AG066707 to F.C.
Footnotes
The statements and opinions expressed in COVID-19 Curbside Consults are based on experience and the available literature as of the date posted. While we try to regularly update this content, any offered recommendations cannot be substituted for the clinical judgment of clinicians caring for individual patients.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.