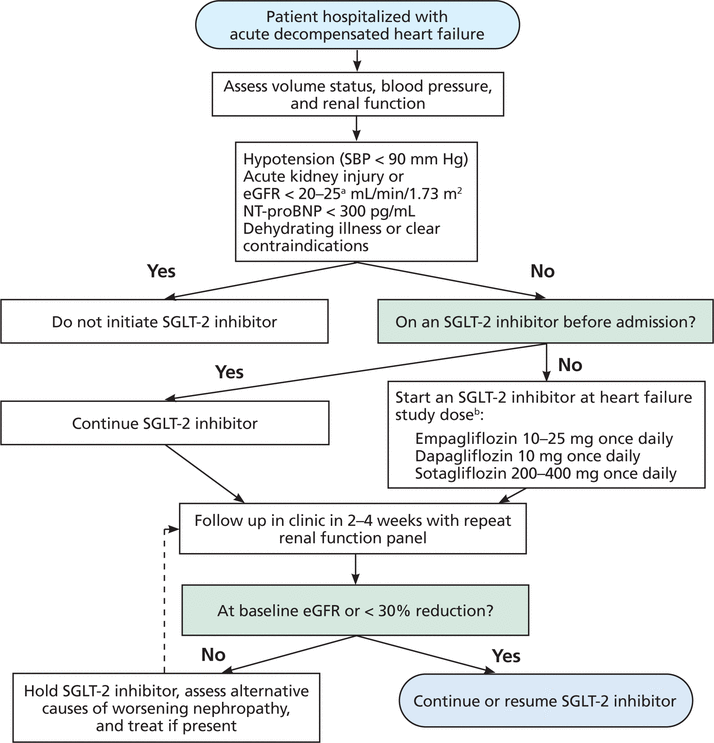
In the January 2024 issue, the article on SGLT-2 inhibitors by Badwan OZ, Braghieri L, Skoza W, Agrawal A, Menon V, and Tang WHW, When should we consider SGLT-2 inhibitors in patients with acute decompensated heart failure? [Cleve Clin J Med 2024; 91(1):47–51. doi:10.3949/ccjm.91a.23034] contained an error in Figure 1. The dosage of empagliflozin was given as 10–25 mg twice daily. The correct dosage is 10–25 mg once daily. The corrected version appears below:
Proposed algorithm for initiating sodium-glucose cotransporter 2 inhibitors in acute decompen-sated heart failure.
a Dapagliflozin: No dosage adjustment for eGFR ≥ 25 mL/min/1.73 m2. Manufacturer labeling does not recommend initiation of therapy at eGFR < 25 mL/min/1.73 m2. Sotagliflozin is not indicated for patients with eGFR < 25 mL/min/1.73 m2. For heart failure, empagliflozin is not indicated for eGFR < 20 mL/min/1.73 m2. For type 2 diabetes mellitus, empagliflozin is not indicated for eGFR < 30 mL/min/1.73 m2.
b Direct evidence on the effects of canagliflozin and ertugliflozin on heart failure outcomes is available only in patients with type 2 diabetes mellitus. It remains to be determined if they have similar effects in patients without type 2 diabetes.
eGFR = estimated glomerular filtration rate; NT-proBNP = N-terminal pro-B-type natriuretic peptide; SBP = systolic blood pressure; SGLT-2 = sodium-glucose cotransporter 2
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.