ABSTRACT
This review focuses on an alternative strategy utilizing small molecules to inhibit a key signal-transduction pathway, the Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway. The JAK-STAT pathway mediates biologic activity for a large number of inflammatory cytokines and mediators.
INTRODUCTION
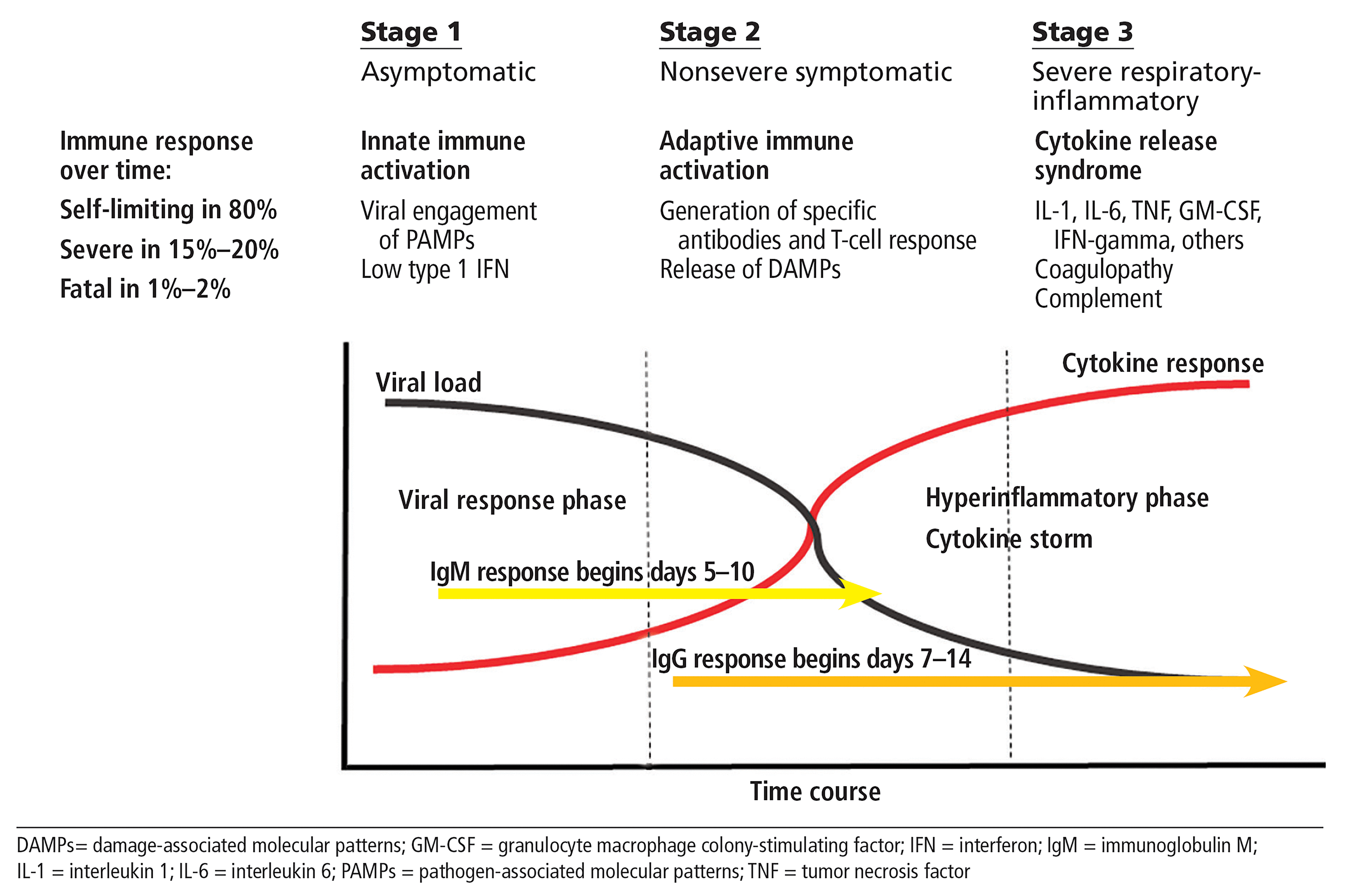
Previous parts of this series focused on the basic immunobiology of severe COVID-19 disease and the role of inflammatory cytokines and their select targeting in an effort to limit respiratory damage, coagulopathy, end-organ failure, and death.1 Figure 1 represents the idealized model in this series, and the current iteration remains relevant, though our granular understanding of immunopathogenesis has progressed. The term “COVID-19 cytokine storm”2 still has relevance, though recently some have focused on meaningful quantitative (especially interleukin 6) and qualitative differences between the inflammatory phase of COVID-19 and inflammatory states observed in other relevant disease states such as acute respiratory distress syndrome. Despite such questioning, enthusiasm remains strong for therapeutic strategies that focus on limiting damaging effects of inflammatory mediators on host tissues. Most biologic therapies (ie, targeted therapies) currently under investigation in COVID-19 employ monoclonal antibodies produced by recombinant technology, capable of selectively attacking inflammatory phase 3, one cytokine at a time.
Course of COVID-19 infection: A paradigm for therapy.
This review focuses on an alternative strategy (ie, targeted synthetic therapies) utilizing small molecules to inhibit a key shared signal-transduction pathway, the Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway. The JAK-STAT pathway mediates biologic activity for a large number of inflammatory cytokines and mediators and has been targeted by several therapeutics, which are now in clinical use across a spectrum of immune-mediated inflammatory diseases.3
SCIENTIFIC RATIONALE FOR JAK-STAT INHIBITION IN COVID-19
The JAK-STAT pathway plays a major role in transferring of signals from cell-membrane receptors to the nucleus and is essential for a wide range of cytokines and growth factors to exercise their biologic activity.3 JAK-STAT activation contributes to a host of critical events, such as hematopoiesis, inflammation, the development of the immune system, and deployment of a variety of effector pathways. Cytokines are one of the major products of cells of innate and adaptive immunity, and more than 60 factors bind to receptors termed type I and type II cytokine receptors and mediate their downstream effects through the JAK-STAT pathway of signal transduction. These cytokines are essential for initiating and orchestrating innate and adaptive immune reactions in health but can be the source of uncontrolled inflammation and tissue damage in the setting of a variety of genetic and acquired immune-mediated diseases including COVID-19.
The lead rationale for utilizing JAK inhibitors in COVID-19 is based on an examination of the cytokines already known to be elevated in advanced COVID-19 disease and that act via the JAK-STAT signaling pathways (Table 1).4 This evaluation suggests that a broad-based inhibitory approach may be beneficial in stage 3 disease (Figure 1). Broad-based inhibition, in contrast to single anti-cytokine based therapies with monoclonal antibodies, is appealing as data supporting the identification of a single cytokine as the clear upstream source of the inflammatory process have been problematic. As proof of concept, the kinase inhibitor, ruxolitinib, has been used successfully to treat refractory cytokine storm in patients with relapsed refractory hemophagocytic lymphohistiocytosis.5
Cytokines of interest in COVID-19 involving the JAK-STAT pathway
A second rationale for employing JAK inhibition in COVID-19 stems from an analysis using artificial intelligence identifying a role for the JAK1 inhibitor baricitinib in inhibiting relevant inflammatory pathways, but also in providing evidence that the drug was capable of inhibiting other non-JAK kinases (ie, numb associated kinases or NAKs), which appear to be involved in viral entry.6 Thus, if this is true, baricitinib may have a structural and mechanistic advantage over other agents in this class because of its capacity to limit both inflammation and viral propagation. The original theoretical work is now supported by mechanistic ex vivo studies and forms the rationale for the investigation of this agent in randomized trials.7
Collectively, the potential for a broad-based immunosuppressive therapy with some antiviral activity is attractive, and the extensive experience of its use in non-COVID-19 immune diseases has also furthered our understanding of how to balance the benefits and risks when using this class of agents.8
CLINICAL EXPERIENCE OF JAK INHIBITION IN COVID-19
To date, investigations examining the use of JAK inhibitors in COVID-19 have been limited mostly to case reports and small retrospective series that have been largely positive. Table 2 is a list (as of January 20, 2021) of registered clinical trials on www.clinicaltrials.gov employing currently approved JAK inhibitors; other experimental JAK inhibitors and multiple non-JAK inhibitors are also being currently investigated.9
Clinical trials involving JAK-inhibitors in COVID-19 (7/20/2020)
A few trials with more than 10 patients have been reported and are instructive. The first, an Italian study, employed baricitinib 4 mg per day for 2 weeks in all consecutive COVID-19 hospitalized patients (from March 16 to March 30, 2020) with moderate pneumonia in combination with lopinavir and ritonavir.10 A group of patients receiving the same background therapies treated before the study date served as controls. Overall, there was significant improvement in clinical and laboratory parameters, none of the patients required mechanical ventilation, and no major safety issues were observed.
In one of the most robust studies reported to date, the JAK1 and JAK2 inhibitor ruxolitinib was compared with standard of care plus placebo in a randomized controlled multicenter trial with 22 patients in the ruxolitinib group and 21 in the control arm.11 Overall, 90% of the active arm showed improvement by chest computed tomography, whereas improvement was seen in only 62% of the control group. Three patients died of respiratory failure in the control arm, no deaths occurred in the ruxolitinib group, and there were no major safety issues.
In a small uncontrolled trial in Germany, 14 patients were followed carefully using a newly developed COVID-19 inflammation score and treated with ruxolitinib over a 14-day period. Overall, 12 of 14 achieved significant reductions in disease activity with no safety signals.12 As typical of most COVID-19 clinical trials, the experimental therapy was often added to other experimental therapies that at the time of investigation were standards of care, thus confounding interpretation of outcomes.
A recent preprint publication of a systematic review of MEDLINE and MedRxiv studies of JAK inhibitors or type I interferons revealed significantly reduced odds of mortality (odds ratio 0.12, 95% confidence interval [CI] 0.03–0.39, P < .001) for JAK inhibitors. This interesting finding suggests a positive therapeutic effect for this class of therapy, but the review is seriously limited by the lack of randomized controlled studies and peer review.13
On November 19, 2020, baricitinib was granted emergency use authorization by the Food and Drug Administration, in combination with remdesivir, to treat suspected or laboratory confirmed COVID-19 in hospitalized adults and pediatric patients at least 2 years of age requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation. The recommended dose for adults is 4 mg per day for 14 days with dose adjustments for renal or hepatic impairment.14
The basis for this approval was the ACTT-2 study, a randomized controlled trial of baricitinib alone versus baricitinib plus remdesivir in hospitalized patients with COVID-19.15 Overall, the effects of this combined regimen were modest with a 1 day shortening of recovery time, median 7 days (95% CI 6–8 days) in the baricitinib arm versus 8 days (95% CI 7–9 days) in the remdesivir-alone arm. The 28 day mortality was 5.1% in the active group versus 7.8% in the control group (Hazard ratio 0.65, 95% CI 0.39–1.09). The effect size was greatest for those requiring noninvasive ventilation or high-flow oxygen and lowest for those without oxygen requirements suggesting that the stage and timing of treatment may be critical, though none of these secondary outcomes achieved statistical significance. Serious adverse events were less frequent in the active group. What remains to be determined is how this therapy compares with dexamethasone, which has become the standard of care for COVID-19 patients with this same degree of clinical severity. A recently launched randomized head-to-head trial of baricitinib and remdesivir versus dexamethasone and remdesivir is underway — the Adaptive COVID-19 Treatment Trial 4 (ACTT-4).16
PRINCIPLES OF SAFETY
The past decade has provided us with a robust database analyzing the safety of the class of JAK inhibitors, and based on this, there are formidable concerns particularly in the areas of infectious and cardiovascular complications. A recent narrative review on safety with JAK inhibitors described an enhanced risk for opportunistic infections and a particularly high frequency of viral infections, especially herpes zoster.8 The relevance of this toxicity to COVID-19 is not apparent, as the mechanisms of maintenance of viral latency to varicella and the integrated host defense against respiratory viral infections are highly different. Still, vigilance for infectious complications is critical in the use of JAK inhibitors in this setting.
A second area of concern is the potential to further increase the risks of hypercoagulable complications, which are already overexpressed in the setting of COVID-19. JAK inhibitors are known to increase the risks of venous thromboembolism in rheumatoid arthritis, though the mechanisms contributing to this phenomenon are unclear. Finally, the clinical application of JAK inhibitors in the setting of COVID-19 also raises concerns regarding off-target effects on integrated antiviral immunity through inhibition of interferon signaling. Type I and III interferon activity is mediated via JAK1, JAK2, and TYK2 and may be vulnerable to off-target effects. This pathway is known to be suppressed in patients with COVID-19, and further suppression could contribute to failure to clear active infection.17
DOSAGE AND ADMINISTRATION
There are currently 3 approved JAK inhibitors under investigation in COVID-19; their indications, dosing, warnings, and side effects are listed in Table 3. All are orally administered once or twice daily and have narrow therapeutic ranges for dosing. In COVID-19 clinical trials, the doses under investigation are generally within the same range used in immune-mediated diseases, with some provisions for dose escalation as part of the protocol.
Current JAK inhibitors
LABORATORY MONITORING
When JAK inhibitors are used in COVID-19, careful laboratory monitoring is important, as the drug class has numerous effects on biochemical and hematologic parameters.8 While there are small differences in adverse effects across agents in this class, presumably reflecting differential JAK selectivity, cytopenias including neutropenia and anemia are often observed. Of note, lymphopenia, observed primarily with tofacitinib, is of concern especially in COVID-19, where this is a biomarker correlated with severe disease.1 Elevations of liver enzymes and perturbations of serum lipids are of concern but are also infrequent, and functional liver impairment with hyperbilirubinemia is rarely observed. Serum creatinine and creatinine kinase may also be adversely affected, but this is rarely severe.
CONCLUSION
JAK inhibitors are a class of drugs that are growing rapidly for the treatment of a wide variety immune-based diseases; their mechanism of action is broadly immunomodulatory, and some also have antiviral potential. Experience with these drugs in non-COVID-19 settings has informed us of potential safety issues, and this will hopefully allow effective risk-mitigation. Baricitinib is currently approved under an emergency use authorization license, though how it will be positioned relative to current use of dexamthasone remains to be determined. Numerous trials of JAK inhibitors underway should soon provide more meaningful data on potential effects in patients with COVID-19.
DISCLOSURES
Dr. Leonard Calabrese has disclosed financial relationships (consulting, teaching, or speaking) with Abbvie Pharmaceuticals, BMS, Crescendo, GSK, Genentech-Roche, Horizon Pharma, Janssen, Novartis, Pfizer, Regeneron, Sanofi Aventis, and USB. Dr. Cassandra Calabrese has disclosed financial relationships (consulting, teaching, or speaking) with Abbvie and Sanofi-Regeneron.
Footnotes
The statements and opinions expressed in COVID-19 Curbside Consults are based on experience and the available literature as of the date posted. While we try to regularly update this content, any offered recommendations cannot be substituted for the clinical judgment of clinicians caring for individual patients.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.