In patients with acute decompensated heart failure (ADHF), the initial diuretic regimen should maximize intravenous loop diuretics based on urine output or spot urine sodium. Combination therapy can be used if diuretic resistance occurs.
ADHF accounts for more than 1 million hospitalizations per year in the United States, and inadequate diuresis is a common cause of readmissions and higher mortality.1 In the absence of cardiogenic shock, ADHF manifestations are driven primarily by expansion of extracellular fluid volume, leading to elevated cardiac filling pressures and congestion (edema, dyspnea, and orthopnea).2,3 Despite the evidence supporting guideline-directed medical therapy, studies guiding diuresis are limited.
LOOP DIURETICS
Loop diuretics are the cornerstone of ADHF management. Their early administration is linked to lower in-hospital mortality rates.2 Furosemide, torsemide, and bumetanide are the most used (Table 1).1,4,5 Torsemide and bumetanide have better bioavailability than furosemide, and data suggest torsemide superiority, given its possible improved outcomes and mitigation of cardiac fibrosis.3
Commonly used diuretics and doses in chronic heart failure
Initial dosing
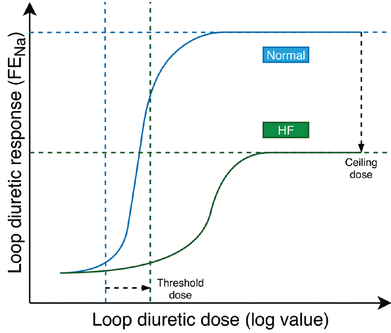
Loop diuretics have a steep dose-response curve with little natriuretic effect until an individualized threshold is reached, and maximum natriuretic effect once the ceiling dose is achieved (Figure 1).2 Dose increases beyond the ceiling may increase the duration of natriuresis rather than the rate by maintaining the diuretic concentration above the threshold for longer. The dose-response relationship is log-linear, meaning the dose should be adjusted in a logarithmic fashion (eg, an increase from 20 mg to 40 mg is greater than 220 mg to 240 mg).3
Loop diuretic dose-response curves in patients with heart failure (green line) and without heart failure (blue line). Heart failure shifts the curve down and to the right, translating to the need for higher doses of diuretics to achieve the same degree of diuresis and decreased maximal diuretic response.
FENa = fractional excretion of sodium
Based on data from reference 2.
Regarding initial dosing, the DOSE trial (Diuretic Optimization Strategies Evaluation)4,6 found better symptom improvement with aggressive intravenous loop diuretic dosing 2.5 times the total daily oral dose compared to a numerically equal dose. There is conflicting evidence about the relationship between diuretic dosing, renal dysfunction, and clinical outcomes.3,7 In general, to avoid premature cessation of diuretic therapy, doubling of creatinine or an increase greater than 1 mg/dL (instead of > 0.3 mg/dL) has been suggested as true renal dysfunction requiring further workup.2
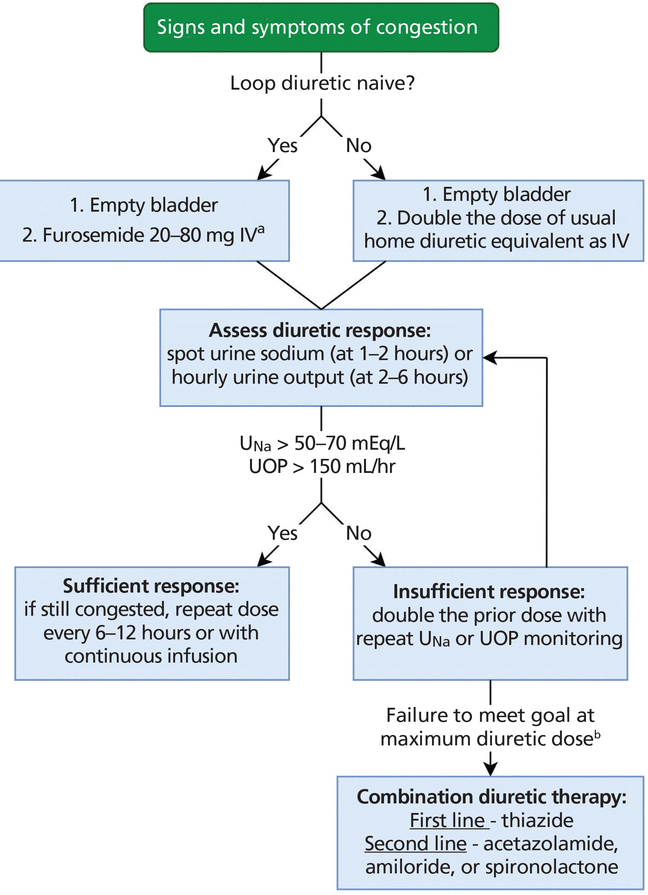
Recent data suggest that using urine output and spot urine sodium to predict short-term responsiveness to intravenous loop diuretics in ADHF permits more timely adjustments to therapy (Figure 2).1–3 With this approach, if the goal of a urine output of more than 150 mL/hour or a urine sodium greater than 50 to 70 mEq/L is not achieved at 2 hours after the initial dose, the dose should be doubled and the parameters rechecked 2 hours after the repeat dose until the goals are met. When the goals are met, the same dose can be administered every 6 to 12 hours until volume overload resolves.3 Continuous furosemide infusion is also commonly used in refractory cases, given favorable pharmacodynamics with concentration maintenance above the threshold. The DOSE trial showed no difference in efficacy between continuous and intermittent dosing, but loading doses were not given at infusion initiation.5
Algorithm for initiation (day 1) of diuretic titration in patients with acute decompensated heart failure.
aHigher dose for reduced glomerular filtration rate.
bSee Table 1 for maximum recommended total daily dosing. IV = intravenous; UNa = urine sodium; UOP = urine output
DIURETIC RESISTANCE
Diuretic resistance is defined as persistent congestion with inadequate response to escalating diuretic doses.1 Although there is no consensus on a precise metric, the inability to meet short-term urine sodium goals (Figure 2) can predict diuretic resistance. The proposed quantitative definition is failure to increase urine sodium by 90 mEq/L despite high-dose oral furosemide (160 mg twice daily or equivalent) over 3 days.7
Loop diuretics achieve their effect primarily by secretion into tubular fluid by proximal organic anion transporters, a process dependent on renal blood flow and serum pH.7 The response to loop diuretics may be diminished due to genetic polymorphisms altering transport and metabolism,8 low absorption from gut edema (as may occur with oral furosemide), low plasma protein content (> 90% protein-bound), and low renal function or perfusion (particularly with nonsteroidal anti-inflammatory drugs or possibly aspirin).3 Dietary sodium restriction nonadherence must also be ruled out since postdiuretic sodium retention can mimic true diuretic resistance.3 Renin-angiotensin system activation may also contribute, but distal tubular cell hypertrophy with increased sodium resorption is emerging as the primary mechanism of diuretic resistance.3
OPTIONS FOR AUGMENTING DIURESIS
Several options exist to augment diuresis. However, most experts recommend delaying combination therapy until loop diuretic dosing is optimized (Figure 2), to avoid risks of renal dysfunction and electrolyte abnormalities.3
First-line therapy: thiazide diuretics
Thiazide diuretics overcome the increased sodium avidity of the distal convoluted tubule that occurs with chronic loop diuretic use.5,9 Commonly used thiazide diuretics are metolazone and hydrochlorothiazide, but other options have similar efficacy and adverse-event rates (Table 1).9 The current guidelines for thiazide use are based on small studies3,9 and the CARRESS-HF trial (Cardiorenal Rescue Study in Acute Decompensated Heart Failure),10 where thiazides used in a stepwise pharmacologic algorithm were compared with ultrafiltration.
Second-line therapy: acetazolamide, potassium-sparing diuretics
Although a poor diuretic on its own, the carbonic anhydrase inhibitor acetazolamide has been shown to augment the loop diuretic effect through decreased sodium bicarbonate reabsorption in the proximal tubules (Table 1). This allows more sodium delivery to the loop of Henle, but tolerance develops after 72 hours.1 Acetazolamide also has intrinsic renal vasodilatory effects and blocks the pendrin system of chloride-bicarbonate exchange in the distal nephron.1,11 Combination therapy with acetazolamide has shown greater decongestion success compared with loop diuretics alone in the recent ADVOR trial (Acetazolamide in Decompensated Heart Failure With Volume Overload),12 without significant differences in adverse events or the secondary end points of death or rehospitalization.
The potassium-sparing diuretics amiloride and triamterene inhibit distal epithelial sodium channels in the collecting duct. Anecdotal evidence suggests amiloride can result in decongestion, but randomized clinical trials are lacking, and these medications can lead to severe hyperkalemia.2 Spironolactone works on a separate receptor to cause mild natriuresis, and it reduces potassium wasting of loop and thiazide diuretics. Data are limited regarding its synergistic use with loop diuretics in ADHF. The ATHENA-HF trial (Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure)13,14 found no difference in outcomes with this regimen; however, the patient sample did not exhibit diuretic resistance, and the 4 days of therapy may have been inadequate for response (spironolactone is a prodrug).
Alternatives
Tolvaptan. Vasopressin levels are increased in heart failure, worsening fluid retention. The selective V2 vasopressin receptor antagonist tolvaptan blocks distal tubule reabsorption of free water. It has been shown to improve filling pressures when combined with loop diuretics. It may be preferable in patients with hyponatremia and kidney dysfunction, but no improved outcomes have been seen.13 A moderate-sized cohort study comparing metolazone, chlorothiazide, and tolvaptan has shown excellent weight loss with no significant difference between the groups.15 Conivaptan has also demonstrated promising diuresis augmentation in heart failure without adverse renal or hemodynamic effects.16
Sodium-glucose cotransporter 2 inhibitors decrease proximal sodium absorption. Strong evidence from clinical trials shows significant diuresis with renoprotection and improved heart failure outcomes, but coadministration with loop diuretics has not been studied.1
Hypertonic saline. Intravenous hypertonic saline has been shown to augment diuresis by improving renal perfusion by osmotic “pulling” of free water into the intravascular space and by improving the loop diuretic effect through better sodium delivery to the loop of Henle.13 It also improves inotropy through myocardial stimulation.1 This method has been associated with reduced mortality rates, hospital length of stay, and treatment cost compared with furosemide alone.1
Ultrafiltration. Methods such as ultrafiltration can be used in congestion that is refractory to medical therapy. Outpatient peritoneal dialysis has been described for advanced heart failure with cardiorenal syndrome.13 However, there is no evidence favoring ultrafiltration over loop diuretics as first-line therapy.2
Hemodynamic evaluation. An invasive hemodynamic evaluation should be considered in hospitalized patients who have refractory symptoms despite adequate diuresis, worsening renal failure with increasing diuretics, or repeated hospitalization.17 Wireless implantable pulmonary artery pressure monitors showed promising results in the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III HF Patients) trial,18 in which patients with persistent symptoms who were randomized to receive a monitor had a 28% relative reduction in heart failure hospitalization rates.
THE BOTTOM LINE
ADHF is a major source of healthcare spending in the United States, with many hospital discharges complicated by readmissions due to inadequate diuresis. The initial goal should always be to maximize loop diuretic therapy using urine output or urinary sodium for guidance. Combination therapy can be used when patients respond poorly to escalating loop diuretic doses.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.