A 71-year-old man was referred to the gastroenterology department for evaluation of 9 months of progressive swallowing difficulties associated with epigastric and chest discomfort.
He was a previous smoker (17 pack-years), with a history of coronary artery disease, hypertension, and cervical spinal stenosis requiring decompressive laminectomy with a postoperative course complicated by episodes of aspiration.
DYSPHAGIA: OROPHARYNGEAL OR ESOPHAGEAL
Difficulty swallowing (dysphagia) can be caused by problems in the oropharynx or in the esophagus. Difficulty initiating a swallow can be thought of as oropharyngeal dysphagia, whereas the intermittent sensation of food stuck in the neck or chest is considered esophageal dysphagia.
Focused questioning can help differentiate oropharyngeal symptoms from esophageal symptoms. For example, difficulty clearing secretions or passing the food bolus beyond the mouth or frequent coughing spells while eating is consistent with oropharyngeal dysphagia and suggests a neurologic cause. Our patient, however, presented with a constellation of symptoms more suggestive of esophageal dysphagia.
When eliciting a history of esophageal symptoms, it is crucial to determine the progression of swallowing difficulty, as well as how it directly relates to eating solids or liquids, or both. Difficulty swallowing solid foods that has progressed over time to include liquids would raise concern for an obstruction such as a stricture, ring, or malignancy. On the other hand, abrupt onset of intermittent dysphagia to both solids and liquids would raise concern for a motility disorder of the esophagus. This patient presented with an abrupt onset of intermittent symptoms to both solids and liquids that was associated with substernal chest pain.
Once coronary disease was ruled out by cardiac biomarker testing, electrocardiography, and a pharmacologic stress test, our patient underwent upper endoscopy, which showed a normal esophageal mucosa without masses or obstruction and no evidence of peptic ulcer disease.
WHAT IS THE NEXT STEP?
When upper endoscopy is negative and cardiac causes and gastroesophageal reflux disease have been ruled out, an esophageal motility disorder should be considered.
1. After obstruction has been ruled out with upper endoscopy, which should be the next step in the investigation of esophageal dysphagia?
A 24-hour pH recording
Barium esophagography
Modified barium swallow
Computed tomography of the chest
Barium esophagography is the optimal fluoroscopic study to evaluate the esophageal phase of the swallow. This study requires the patient to swallow a thick barium solution and a 13- mm barium pill under video analysis. It is useful early in the investigation of esophageal dysphagia because it can potentially reveal areas of esophageal luminal narrowing not detected endoscopically, as well as detail the rate of esophageal emptying.1
The modified barium swallow, which is performed with the assistance of a speech pathologist, is similar but only shows the oropharynx as far as the cervical esophagus. Therefore, it would be the best fluoroscopic test to assess patients with possible aspiration or oropharyngeal dysphagia, whereas barium esophagography would be the test of choice in evaluating esophageal dysmotility or mechanical obstruction.
pH testing may be helpful in diagnosing gastroesophageal reflux disease but is less helpful in the evaluation of dysphagia.
Computed tomography of the chest may be useful to evaluate for extrinsic compression of the esophagus, but it is not the best next step in the evaluation of dysphagia.
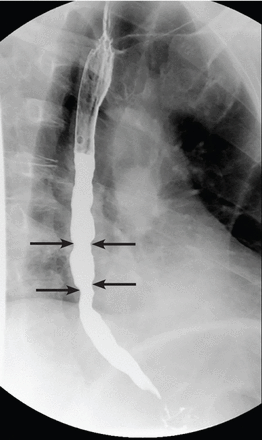
Our patient underwent barium esophagography, which revealed tertiary contractions in the mid and distal esophagus with slight narrowing of the lower cervical esophagus (Figure 1). (Primary contractions are elicited when initiating a swallow that propels the food bolus through the esophagus, while secondary contractions follow in response to esophageal distention to move all remaining esophageal contents from the thoracic esophagus. Tertiary contractions are abnormal, nonpropulsive, spontaneous contractions of the esophageal body that are initiated without swallowing.2)
Barium esophagography showed tertiary contractions (arrows) in the distal esophagus.
EOSINOPHILIC ESOPHAGITIS
Histologic study of biopsies of the mid and distal esophagus from our patient’s upper endoscopy revealed 5 eosinophils per high-power field.
2. Does this patient meet the criteria for the diagnosis of eosinophilic esophagitis?
Yes
No
No. Having eosinophils in the esophagus is not enough to diagnose eosinophilic esophagitis, as eosinophils are also common in patients with gastroesophageal reflux disease.
Eosinophilic esophagitis is defined as a chronic immune-mediated esophageal disease with histologically eosinophil-predominant inflammation (with more than 15 eosinophils per high-power field). The diagnosis is additionally based on symptoms and endoscopic appearance.3 When investigating possible eosinophilic esophagitis, it is recommended that 2 to 4 samples be obtained from at least 2 different locations in the esophagus (eg, proximal and distal), because the inflammatory changes can be patchy.
WHAT DOES THE PATIENT HAVE?
3. What is the likely cause of this patient’s dysphagia?
Eosinophilic esophagitis
Achalasia
Esophageal spasm
Extrinsic compression
Esophageal malignancy
Eosinophilic esophagitis causes characteristic symptoms that include difficulty swallowing, chest pain that does not respond to antisecretory therapy, and regurgitation of undigested food. As we discussed above, this patient has only 5 eosinophils per high-power field and does not meet the histologic criteria for eosinophilic esophagitis.
Achalasia has a characteristic “bird’s beak” appearance on esophagography that results from distal tapering of the esophagus to the gastroesophageal junction,1 and this is not apparent on our patient’s study.
Review of this patient’s esophagogram also does not reveal any extrinsic compression, esophageal malignancy, or distal tapering suggesting achalasia. In light of the abrupt onset of symptoms related to both solids and liquids associated with atypical chest pain, the primary concern should be for esophageal spasm.
ONE MORE TEST
4. What study would you order next to better elucidate the cause of this patient’s esophageal disorder?
High-resolution esophageal manometry
Esophagogastroduodenoscopy (EGD) with endoscopic ultrasonography
24-hour pH and impedance testing
Wireless motility capsule
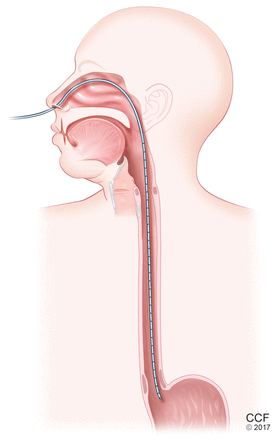
Esophageal manometry (Figure 2) is used to evaluate the function and coordination of the muscles of the esophagus, as in disorders of esophageal motility.
Esophageal manometry involves passing a probe with pressure sensors through the nose and down the esophagus to the level of the lower esophageal sphincter. As the patient swallows, the probe senses the wave of contraction, which can be graphed to assess the motor function of the esophagus (see Figure 3).
High-resolution manometry is the gold standard for evaluation of esophageal motility. It is appropriate in evaluating dysphagia or noncardiac chest pain without evidence of mechanical obstruction, ulceration, or inflammation.4,5
High-resolution manometry differs from conventional manometry in that the catheter has more sensors to measure intraluminal pressure (36 rather than the usual 7 to 12). The data are translated into pressure topography plots (Figure 3).6,7
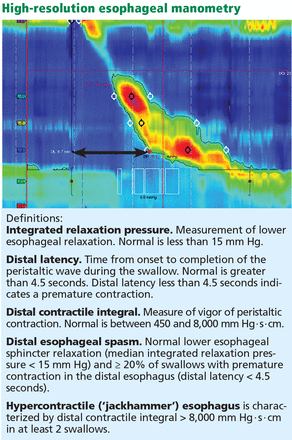
In esophageal manometry, the vertical axis shows the length along the esophagus, the horizontal axis represents time, and the color code depicts pressure, from blue (low) to red (high). This study shows a normal distal latency (black arrow) of 6.7 seconds, and a normal distal contractile integer of 2,300 mm Hg · s · cm.
Updated guidelines on how to interpret the findings of high-resolution manometry are known as the Chicago 3.0 criteria.4 According to this system, esophageal motility disorders are grouped on the basis of lower esophageal sphincter relaxation and then further subdivided based on the character of peristalsis.
EGD with endoscopic ultrasonography would not be appropriate at this time because there is little suspicion of an extraluminal mass that needs to be investigated.
A 24-hour pH and impedance study is helpful in determining the presence of esophageal acid exposure in patients presenting with gastroesophageal reflux disease. This patient does not have symptoms of heartburn or regurgitation; therefore, this investigation would not be of value.
A wireless motility capsule would help in investigating gastric and small-bowel motility and may be useful in the future for this patient, but at this point it would provide little additional utility.
ESOPHAGEAL SPASM
Our patient underwent high-resolution esophageal manometry. The results (Figure 4) revealed a normal resting pressure in the lower esophageal sphincter and complete relaxation in all swallows. The body of the esophagus demonstrated premature contractions in 90% of swallows. Overall, these findings were consistent with the diagnosis of distal esophageal spasm.
In our patient, esophageal manometry showed distal esophageal spasm and premature contraction, with a distal latency (black arrow) less than 4.5 seconds.
TREATMENTS FOR ESOPHAGEAL SPASM
In addition to incorporating data obtained from endoscopy, esophagography, and manometry, it is crucial to identify the patient’s predominant symptom when planning treatment. For example, is the prevailing symptom dysphagia or chest pain? Additional consideration must be given to medical, surgical, and psychiatric comorbidities.
5. Which of the following is appropriate medical therapy for esophageal spasm?
Calcium channel blockers
Nitrates
Hydralazine
Phosphodiesterase 5 (PDE5) inhibitors
All of the above
All of these have been used to treat distal esophageal spasm as well as other hypercontractile esophageal motility disorders.8–20
Calcium channel blockers have proven to be effective in randomized controlled trials. Diltiazem has been shown to be beneficial at doses ranging from 60 to 90 mg, as has nifedipine 10 to 20 mg 3 times daily. Although different drugs of this class tend to relax the lower esophageal sphincter to different degrees, when choosing among them in patients with hypercontractile disorders there is little concern for potentially precipitating reflux.8–13
Nitrates, hydralazine, and PDE5 inhibitors have been effective in uncontrolled studies but have not been studied in randomized trials.14–17
Other treatments. Patients may also benefit from neuromodulators such as trazodone and imipramine for chest pain and optimization of antisecretory therapy if they have concomitant gastroesophageal reflux disease.18–20
Patients who have documented esophageal hypercontractility along with reflux disease confirmed by an abnormal pH study show significant improvement in their chest pain symptoms with high doses of a proton pump inhibitor (PPI). As our patient presented with chest pain and dysphagia, a dedicated pH study was not needed, and we could progress straight to manometry and a trial of a PPI.
Our patient was started on a PPI and nifedipine but developed a pruritic rash. As rash does not preclude using another medication in the same class, his treatment was changed to diltiazem 30 mg by mouth 3 times a day, and his dysphagia improved. However, he continued to experience intermittent chest pain with swallowing. After discussion of neuromodulator therapy, he declined additional pharmacologic therapy.
A NONPHARMACOLOGIC TREATMENT?
6. Which of the following would you offer this patient as a nonpharmacologic alternative for his esophageal pain?
St. John’s wort
Ginkgo biloba
Ginseng
Peppermint extract
Eucalyptus oil
In a small, open-label study in patients with esophageal spasm, the use of 5 drops of commercially available 11% peppermint extract in 10 mL of water significantly decreased simultaneous contractions and resolved chest pain.21 Esophageal manometry was performed 10 minutes after the peppermint solution was consumed, and the results showed improvement in esophageal spasm. While the authors of this study did not make any formal recommendations, the findings suggest that peppermint extract should be given 10 minutes before meals.
There is no evidence for or against the use of the other nonpharmacologic treatments mentioned here.
PAIN RELIEF
7. If a pharmacologic approach were chosen, which would be the best option for pain relief in this patient?
Oxycodone 5 mg every 8 hours
Acetaminophen 650 mg every 8 hours
Ibuprofen 400 mg every evening at bedtime
Trazodone 100 mg every evening at bedtime
Imipramine 50 mg every evening at bedtime
Aripiprazole 5 mg by mouth every day
Trazodone would be the most appropriate of these options. Doses of 100 mg to 150 mg every evening at bedtime have been shown to significantly improve global assessment scores of pain at 6 weeks.18
Imipramine 50 mg every evening at bedtime would be another option and also has been shown to reduce chest pain.19
Even though these were the doses that were investigated, in clinical practice it is common to start at lower doses (trazodone 50 mg or imipramine 10 mg) and to then titrate every 4 weeks based on the patient’s response.
Opiates (eg, oxycodone) should be avoided, as they can cause esophageal motility disorders such as spasm or achalasia.22
Acetaminophen and aripiprazole have not been studied exclusively for their effect on chest pain related to esophageal spasm.
RECURRENT SYMPTOMS
The patient’s dysphagia initially decreased while he was taking diltiazem 30 mg 3 times a day, but it recurred after 6 months. The dosage was increased to 60 mg 3 times a day over the course of the next year, with minimal response. (The maximum dose is 90 mg 4 times a day, but because of side effects of lightheadedness and dizziness, out patient could not tolerate more than 60 mg 3 times a day).
ENDOSCOPIC THERAPY
8. What endoscopic therapies are appropriate for patients with esophageal spasm that does not respond to medication?
Bougie dilation
Balloon dilation
Onabotulinum toxin injection
Expandable mesh stent placement
Mucosal sclerotherapy
Onabotulinum toxin injections have been shown to improve dysphagia when given in a linear pattern.23
Endoscopic dilation has not been shown to be beneficial in this setting, as a study found no difference in efficacy between therapeutic (54-French) and sham (24-French) bougie dilation.24
Our patient received 100 units of onabotulinum toxin (10 units every centimeter in the distal 10 cm of the esophagus). Afterward, he experienced resolution of dysphagia, with only mild intermittent chest pain, which was controlled by taking peppermint extract as needed. The symptoms returned approximately 1 year later but responded to repeat endoscopy with onabotulinum toxin injections.23,25
Peroral endoscopic myotomy
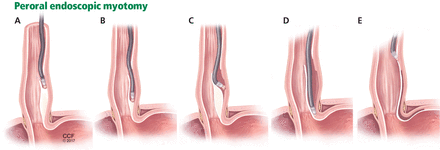
Another relatively new endoscopic treatment for esophageal motility disorders is peroral endoscopic myotomy (Figure 5). During this procedure a tiny incision is made in the esophageal mucosa, permitting the endoscope to tunnel within the lining. The smooth muscle of the distal esophagus and lower esophageal sphincter is then cut, thereby freeing either the spastic muscle (in distal esophageal spasm) or the hyperactive lower esophageal sphincter (in achalasia).26,27
In peroral endoscopic myotomy, an experimental treatment for esophageal spasm, the endoscope is inserted into the space between the endothelium and muscle (A) and advanced to the distal esophagus (B or C) or the lower esophageal sphincter (D), where the muscle is severed. The endoscope is then withdrawn (E).
In an open trial, after undergoing peroral endoscopic myotomy for esophageal spasm and hypercontractile esophagus, 89% of patients had complete relief of dysphagia, and 92% had palliation of chest pain.28 Of note, the rate of relief of dysphagia was higher for patients with achalasia (98%) than for non-achalasia patients (71%).
- Copyright © 2017 The Cleveland Clinic Foundation. All Rights Reserved.