ABSTRACT
Concern for contrast-induced acute kidney injury (CI-AKI) or nephrogenic systemic fibrosis may lead to withholding important studies from patients with kidney disease. However, the actual risk or even the existence of these conditions has recently been called into question. The truth probably lies somewhere in the middle.
The risk of CI-AKI appears to be highest in patients with the lowest kidney function, but the overall risk is lower than initially thought.
In the absence of an equivalent alternate study, iodinated contrast studies that are thought to be crucial to the care of patients with kidney disease should not be withheld out of concern for CI-AKI.
Volume expansion with isotonic fluid appears to be the only intervention with a possible benefit in preventing CI-AKI. This is recommended in high-risk patients unless they are clinically volume-overloaded.
With the highly stable class II gadolinium-based contrast agents, the risk of nephrogenic systemic fibrosis appears to be extremely low and as such safe even for patients with advanced, predialysis kidney disease.
End-stage kidney disease patients on dialysis do require a hemodialysis treatment immediately after gadolinium administration.
Contrast-induced acute kidney injury (CI-AKI) and nephrogenic systemic fibrosis (NSF) have been 2 of the most feared adverse effects of iodinated contrast media for computed tomography (CT) and gadolinium-based contrast media for magnetic resonance imaging (MRI), respectively. Newer and safer contrast agents and, perhaps, better patient selection and prophylactic measures have ameliorated those risks. Recently, some authors have suggested that NSF has been eradicated, while others question whether CI-AKI is an actual entity.
This review presents and evaluates the data around CI-AKI and NSF and critically highlights the most recent practice guidelines.
IODINATED CONTRAST AND ‘RENALISM’
Iodinated contrast media are commonly used in modern medicine both intravenously with CT studies and arterially during angiographic procedures. Among the possible adverse effects is acute kidney injury, first reported in the 1950s in patients undergoing intravenous pyelography.1 In the 1980s, larger series of cases of acute kidney injury following coronary angiography were reported, and the term contrast-induced nephropathy was coined.2 With growing attention, it was said to be one of the most common causes of hospital-acquired acute kidney injury,3 contributing significantly to incident chronic kidney disease, end-stage kidney disease, and death.4
Early publications defined contrast-induced nephropathy as an increase in creatinine of 0.5 mg/dL or more, or a 25% increase from baseline within 2 to 5 days of exposure.
In 2012, the Kidney Disease Improving Global Outcomes Working Group suggested the term CI-AKI and defined it as a 50% increase in creatinine from baseline within 7 days of exposure or a 0.3 mg/dL increase within 48 hours.5 CI-AKI is now the accepted terminology to describe kidney injury precipitated by iodinated contrast media.
Presentation
CI-AKI usually presents within 24 to 48 hours of exposure to iodinated contrast media, with elevation in creatinine and, rarely, oliguria. The creatinine level peaks by 3 to 5 days and usually returns to baseline by 7 to 10 days. Sediment analysis shows granular casts and tubular epithelial cells, and the fractional excretion of sodium is usually low.
Risk factors include chronic kidney disease, diabetes, proteinuria, volume depletion, and concomitant exposure to other nephrotoxins. Procedure-related factors include higher-osmolality contrast media, higher volume given, multiple administrations of iodinated contrast media, and intra-arterial administration with first-pass effect.2,6
The diagnosis is clinical, and it is prudent to rule out other causes of acute kidney injury, in particular, atheroembolic kidney disease in patients undergoing angiography with iodinated contrast media.7 While the true incidence of atheroembolic kidney disease compared with that of CI-AKI in this situation is not known, supporting evidence comes from reports demonstrating a correlation between the risk of acute kidney injury and atheroma burden,8 and a lower risk with radial than with femoral angiographic procedures.9 This disease has a very different clinical course but is commonly misdiagnosed as CI-AKI.
Pathophysiologic basis
The pathophysiologic basis for CI-AKI is still not completely understood, but direct and indirect mechanisms have been suggested.10
Iodinated contrast media are directly toxic to the tubular epithelial cells, leading to loss of polarity (loss of channel restriction to either luminal or basolateral membranes) and eventual apoptosis and necrosis. Elevated blood osmolality due to the contrast media, increased viscosity of the luminal fluid, and free radical formation have also been implicated in direct toxicity.7,8
Deranged hemodynamics underlie the indirect adverse effects of iodinated contrast media, with a brief initial vasodilatory state followed by pronounced and sustained vasoconstriction. Prolonged vasoconstriction, which appears to be mediated through alterations in endothelin, nitric oxide, adenosine, and prostaglandin levels, eventually leads to medullary ischemia. Tubuloglomerular feedback has also been postulated as an explanation for the drop in glomerular filtration rate observed in CI-AKI.
Is it all a myth?
Over the past decade, a number of large epidemiologic studies suggested that acute kidney injury following exposure to iodinated contrast media is not necessarily caused by the contrast media. Some reports even questioned whether it is a real disease.11 This has sparked much debate and led to newer names for the phenomenon, including postcontrast acute kidney injury and contrast-associated acute kidney injury (Table 1). The rationale of these new definitions is to eliminate the causality associated with the term CI-AKI.
Nomenclature and definitions of kidney injury related to iodinated contrast media
Whether one believes CI-AKI is real or a myth, this debate is not merely theoretical because conclusions drawn have significant implications for the care of our patients who have chronic kidney disease. For example, Chertow et al12 reported an inappropriately low rate of cardiac angiographic procedures in patients who have chronic kidney disease. Presumably, procedures were withheld out of concern for CI-AKI. They coined the term “renalism” to indicate the perhaps inappropriate attention to the kidneys while ignoring the bigger picture. Although it is not yet reported, one could presume the notion of avoidance may encompass all contrast-enhanced CT studies in the chronic kidney disease population.
Those who question the diagnosis of CI-AKI point to studies reporting similar rates of acute kidney injury in patients undergoing contrast-enhanced CT compared with those undergoing an unenhanced study. Davenport et al13 used a 1:1 propensity matching algorithm and retrospectively reviewed over 17,000 patients who underwent contrast-enhanced CT or unenhanced CT. In patients whose estimated glomerular filtration rate (eGFR) was less than 30 mL/min/1.73 m2, the rate of acute kidney injury was significantly higher in those exposed to contrast (36.4% vs 19.4%, odds ratio 2.96, 95% confidence interval 1.22–7.17). In those with eGFRs of 30 to 59 mL/min/1.73 m2 rates were numerically higher with contrast than without contrast, but the difference did not reach statistical significance, and rates were the same with or without contrast in those with eGFRs of 60 or higher.
McDonald et al14 and, more recently, Hinson et al15 performed similar large epidemiologic propensity-controlled studies showing no difference in rates of acute kidney injury between contrast recipients and those who underwent unenhanced CT. Notably, both studies demonstrated no difference regardless of the definition of acute kidney injury or eGFR stratification. However, patients with eGFRs less than 45 mL/min/1.73 m2 were significantly underrepresented in these studies, accounting for only 5% to 10% of participants, with some studies completely excluding patients whose creatinine was above 4 mg/dL.15
Does that mean that CI-AKI does not exist? We believe that would be an erroneous conclusion. Despite the complex algorithms used in the propensity matching, a selection bias remains as to who undergoes contrast CT and who does not. Clinicians’ perceptions of risks and consequently their decisions to give or withhold contrast cannot be ascertained from retrospective analyses. In addition, prevention strategies, or lack thereof, are not accounted for in these large database-driven studies. Moreover, as stated previously, patients with severely decreased eGFR, who are at highest risk of CI-AKI, were underrepresented in the propensity score studies.
However, the risks of CI-AKI are probably overstated. Initial descriptive studies were mostly uncontrolled, and rates of acute kidney injury were based mostly on ICD codes with little adjudication as to the cause. This would ultimately inflate the rates of acute kidney injury attributed to the iodinated contrast media.16,17 In addition, changing practices, such as prophylaxis, minimizing exposure, and the development of less toxic, lower-osmolar iodinated contrast media have probably played an important role in reducing the rates of CI-AKI.
Nevertheless, CI-AKI remains real. A recent meta-analysis with more than 1,500 patients undergoing peripheral angiography found a higher incidence of acute kidney injury with iodinated contrast media than with carbon dioxide contrast (11% vs 4%, respectively.18 In addition, our group recently published a propensity-matched study evaluating rates of acute kidney injury in patients with stage 3 or 4 chronic kidney disease undergoing coronary angiography, contrast-enhanced CT, or nonenhanced CT.19 Postcontrast acute kidney injury was noted in 27%, 24%, and 24% of patients, respectively. All cases of acute kidney injury were then adjudicated by 2 nephrologists through chart review to ascertain the cause. They found that the incidence of CI-AKI was 16.5% in the coronary angiography group and 12.5% in the contrast-enhanced CT group.
Therefore, despite the lack of conclusive data, CI-AKI remains very much a real entity, although the incidence is lower than originally thought.
The evidence, or lack of evidence, for preventive strategies
The evidence regarding strategies to prevent CI-AKI is far from satisfying. Hiremath and Velez16 described it as “a proliferation of small, underpowered trials, often with interventions that were poorly thought out” and said that “subsequent meta-analyses have spawned meta-confusion.” With that in mind, we will try to critically evaluate some of the proposed prophylactic interventions.
Volume expansion
Solomon et al20 first reported volume expansion with 0.45% saline to be effective in preventing CI-AKI. Mueller et al,21 analyzing 1,620 patients, reported a lower incidence of acute kidney injury with periprocedural use of isotonic saline than with 0.45% saline.
Although hydration has become the accepted standard, the recent AMACING trial challenged its role in preventing CI-AKI. Nijssen et al22 randomized 660 patients undergoing contrast-enhanced procedures to undergo volume expansion with 0.9% normal saline or no volume expansion. The latter was found to be noninferior to saline, but the overall rates were low. Notably, patients with an eGFR less than 30 mL/min/1.73 m2 were excluded from the study.
More recently, Timal et al23 performed a randomized multicenter trial in 523 patients with stage 3 chronic kidney disease undergoing contrast-enhanced CT. Randomization to no hydration was noninferior to prehydration with bicarbonate in terms of postcontrast acute kidney injury, with event rates of 2.7% vs 1.5% respectively (relative risk 1.7, 95% CI 0.5–5.9). Noninferiority was also shown on subgroup analyses based on age, eGFR (30–44 vs 45–60 mL/min/1.73 m2) alone or in combination with risk factors including diabetes. However, the event rate in this trial was lower than in previous trials, and therefore, caution should be used with interpreting the results.
The type of fluid used for volume expansion has also been a topic of debate, with bicarbonate-based hydration protocols proposed. The premise is that urinary alkalinization would ameliorate the direct toxicity of iodinated contrast media by decreasing oxygen free-radical generation.10
Multiple small trials and subsequent meta-analyses provided highly divergent results until the Prevention of Serious Adverse Events Following Angiography (PRESERVE) trial put this discussion to rest.24 This large 2-by-2 factorial study randomly assigned 5,177 patients undergoing nonemergency angiography to receive isotonic sodium bicarbonate vs isotonic saline as well as oral acetylcysteine vs placebo. The trial was stopped early due to futility, with acute kidney injury rates of 9.5% in the bicarbonate group and 8.3% in the saline group (P = .13). Therefore, there is no additional benefit to bicarbonate-based hydration compared with isotonic saline.
Pharmacotherapy
Acetylcysteine. The acetylcysteine story mirrors that of bicarbonate: a multitude of small studies followed by a series of meta-analyses yielding conflicting results. However, 2 studies over the past few years should settle this discussion for good: the Coronary and Peripheral Vascular Angiography (ACT) trial,25 with 2,308 patients undergoing an intravascular angiographic procedure randomized to acetylcysteine vs placebo, and the previously mentioned PRESERVE trial.24 Both trials showed no difference in rates of acute kidney injury between the acetylcysteine and placebo groups.
Statins have been postulated to reduce the risk of CI-AKI because of their pleiotropic anti-inflammatory and antioxidant effects, which help stabilize plaque. There have been many conflicting studies, with recent meta-analyses suggesting a possible benefit in patients undergoing coronary angiography.10 Whether this benefit is due to prevention of CI-AKI or atheroembolic kidney disease is not clear. Most patients who undergo coronary angiography ultimately receive high-dose statin therapy anyway, making this a moot point.
Other interventions, including vitamin C, high-flow oxygen, and ischemic preconditioning are promising but the evidence remains lacking.
In summary, volume expansion with isotonic saline appears to be the only intervention with a possible benefit in preventing CI-AKI. This is probably important in patients deemed to be at intermediate to high risk (Table 2). Acetylcysteine has no role as a prophylactic measure, and bicarbonate-based fluids do not appear to offer an added benefit beyond volume expansion. Other preventive measures include using low- or iso-osmolar contrast media with the lowest necessary total dose.
Iodinated contrast media in patients with kidney disease: Key points from the ACR-NKF consensus statement
We also advocate withholding nonsteroidal anti-inflammatory drugs, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers in high-risk patients, acknowledging that the data in that regard are insufficient.
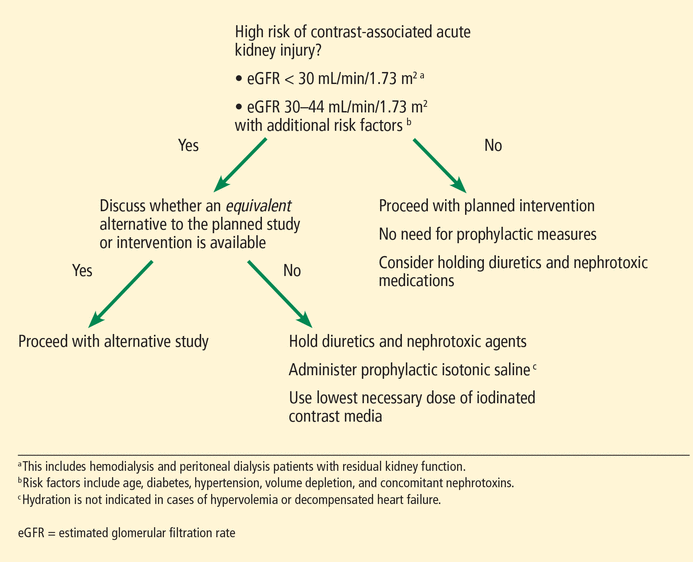
Figure 1 shows our approach when patients with chronic kidney disease require iodinated contrast media.
Our approach to chronic kidney disease patients requiring iodinated contrast media.
Note that we generally include nonanuric patients undergoing hemodialysis or peritoneal dialysis in our high-risk category, and unless they are clinically hypervolemic, we recommend prophylaxis to preserve residual kidney function. A reanalysis of the Canada-USA (CANUSA) peritoneal dialysis study26 elegantly demonstrated that being able to produce as little as 250 mL of urine per day was associated with a 36% lower relative risk of death in peritoneal dialysis patients.
Although the data are less robust, this observation likely applies to hemodialysis patients as well, thus underscoring our recommendation for prophylaxis.27 We emphasize that the goal of hydration in this nonanuric dialysis population is not to make them hypervolemic, and as such, hydration should be forgone in overtly volume-overloaded patients.
The ideal hydration protocol for prevention remains uncertain, and various volume-expansion algorithms have been suggested using fixed or weight-adjusted regimens. Our practice is to give 1 to 1.5 mL per kg per hour starting 1 hour before and continuing for 6 hours after exposure to iodinated contrast media.
Updated recommendations
In response to the changing evidence, the American College of Radiology and the National Kidney Foundation released a joint consensus statement this year28 on the use of intravenous iodinated contrast media in patients with kidney disease. Key points are presented in Table 2.
Future directions
Despite decades of research on iodinated contrast and kidney injury, many questions are yet to be answered. What is the exact mechanism of CI-AKI? What is its true incidence with intravenous vs arterial administration? What significance, if any, does CI-AKI carry?
In our aforementioned study,19 cases adjudicated to be CI-AKI carried no mortality risk, with an overall survival rate similar to that in patients who did not have acute kidney injury. Adjudication is key. We need clear definitions that capture CI-AKI clearly and distinctly from all the potential noise associated with other causes of postcontrast acute kidney injury.
The concept of “renalism” has not only led to fewer angiographic procedures being performed in the chronic kidney disease population,13 it probably also underlies the reason why patients with advanced chronic kidney disease were underrepresented in the observational cohorts described above. Studies need to target this high-risk cohort to better delineate the risks and better establish the utility, or futility, of the currently practiced prophylactic measures. Additional work is clearly needed.
GADOLINIUM-INDUCED NEPHROGENIC SYSTEMIC FIBROSIS
NSF is a debilitating and often-fatal fibrosing disease characterized by skin thickening and organ fibrosis.29 It was first reported in 15 dialysis patients in San Diego in the year 2000.30 However, the relationship between NSF and the use of gadolinium as contrast during MRI remained obscure for a long time, finally being suggested 6 years later in Europe.31
The postulated mechanism was the deposition of toxic free gadolinium molecules in the tissues32 with subsequent increases in circulating fibrocytes,33 an increase in the expression of transforming growth factor beta 1,34 and release of proinflammatory and profibrotic cytokines.35 Eventually, gadolinium was detected by electron microscopy on a skin biopsy specimen, specifically in areas of calcium phosphate deposition in blood vessels.36
By 2009, the disease was well established, and the US Food and Drug Administration (FDA) had received over 500 reports, most of them from the United States37 and Denmark.38
In response to this crisis, the authorities and radiology societies were quick to react. In 2007, both the FDA39 and the European Medicine Agency40 issued warnings highlighting the risk of NSF associated with the use of gadolinium-based contrast agents. The American College of Radiology,41 European Society of Urogenital Radiology,42 and other radiology societies published guidelines and recommendations on how to use gadolinium-based contrast agents, particularly in patients with kidney disease.
Gadolinium agents that have a linear molecular shape pose a higher risk, and their use was contraindicated in patients with acute and severe chronic kidney disease with eGFRs less than 30 mL/min/1.73 m2, as well as in patients on dialysis. Additionally, evidence that gadolinium-based contrast agents are removed with dialysis43 prompted clinicians to change their clinical practice by offering dialysis to patients with advanced kidney dysfunction who were exposed to these agents.
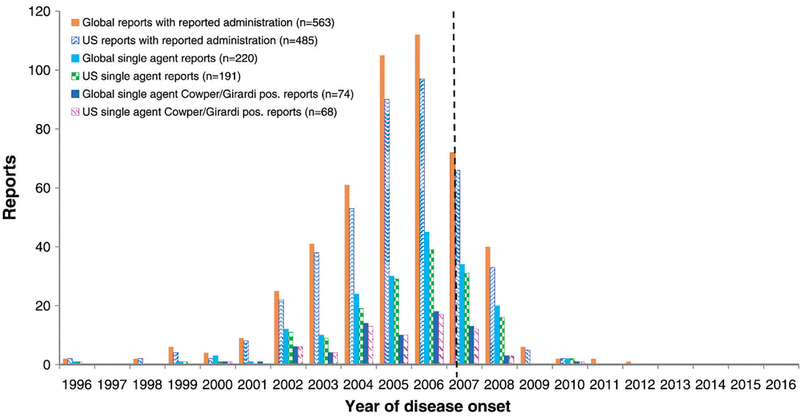
As a result of those measures, the number of cases of NSF was drastically reduced. The last reported case in the United States dates back to 2010, and the last report in the world was in 2012 (Figure 2).44
Number of cases of nephrogenic systemic fibrosis associated with gadopentetate dimeglumine in the United States and around with world, by year of disease onset. Vertical dotted line indicates the introduction of the boxed warning by the US Food and Drug Administration in May 2007.
Endrikat J, Dohanish S, Schleyer N, Schwenke S, Agarwal S, Balzer T. 10 Years of nephrogenic systemic fibrosis: a comprehensive analysis of nephrogenic systemic fibrosis reports received by a pharmaceutical company from 2006 to 2016. Invest Radiol 2018; 53(9):541–550. https://journals.lww.com/investigativeradiology/fulltext/2018/09000/10_years_of_nephrogenic_systemic_fibrosis__a.5.aspx
Classification of gadolinium-based contrast agents
Gadolinium-based contrast agents have been used since the 1980s and were initially thought to have an excellent safety profile.45 This led to their liberal and preferential use compared with iodine-based agents, particularly in patients with reduced kidney function.46 However, their incriminating role in NSF highlighted their potential toxicity.
Gadolinium-based contrast agents share a common structure, with a central heavy metal ion (gadolinium) bound tightly by an organic ligand to form a stable complex, thus minimizing the potential natural toxicity of the free metal ion.47 To avoid gadolinium toxicity, these agents should be highly stable so the gadolinium does not dissociate. Their stability is conferred by their chemical structure, namely whether they are linear or cyclic and whether they are charged (ionic) or electrically neutral (nonionic).48 It is generally recognized that macrocyclic and ionic structures are more stable than linear and nonionic ones.49 Thus, in highly stable agents, gadolinium dissociation is minimized and so is the risk of NSF.
On the basis of their NSF risk (and specifically on the numbers of unconfounded single-agent cases of NSF recorded for each agent), the 9 available gadolinium-based contrast agents are grouped into 3 groups (Table 3)41:
Group I—agents associated with the greatest number of NSF cases.
Group II—agents associated with few, if any, unconfounded cases of NSF.
Group III—agents for which data are limited.
Gadolinium-based contrast agents and risk of nephrogenic systemic fibrosis
It is generally accepted that groups I and III should be avoided in patients with advanced chronic kidney disease.
Risk of NSF today
The guidelines set by the FDA and the radiology societies were undoubtedly effective in curbing the disease and eventually eliminating it. A recent review of 639 patients with biopsy-proven NSF from 173 articles estimated that the risk of NSF per million exposures had decreased from 2.07 before 2008 to 0.028 afterward.50
Most cases were associated with exposure to group I agents. However, those guidelines were applied to all gadolinium-based contrast agents without considering their stability or association with NSF. The downside of this approach was the denial of clinically indicated contrast-enhanced MRI in patients with severe kidney disease, with a subsequent potential real (though unmeasured) harm resulting from misdiagnosis or diagnostic delay.51
In recent years, evidence has been accumulating as to the safety of group II agents. A recent systematic review and meta-analysis evaluated the pooled risks of NSF in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent.52 The authors analyzed 16 studies with 4,931 patients who received group II agents. The pooled risk of NSF was 0% (upper bound of 95% CI 0.07%). Thus, they estimated the per-patient risk of NSF from receiving group II gadolinium-based contrast agents in stage 4 or 5 chronic kidney disease to be less than 0.07%.
This risk is much smaller than that of contrast-induced nephropathy in patients with advanced chronic kidney disease who receive iodinated contrast,53 and thus argues for a better safety profile of contrast-enhanced MRI using group II agents. In fact, the risk appears to be comparable to that of developing a severe allergic reaction to contrast agents, which is estimated at 0.04% for low-osmolality iodinated contrast agents54 and 0.002% to 0.006% for group II agents.55
Updated recommendations
On the basis of accumulating evidence,56–60 the recent guidelines of the American College of Radiology,41 the European Society of Urogenital Radiology,61 and the Canadian Association of Radiologists48 all permit the use of group II gadolinium-based contrast agents in patients with advanced kidney disease. The American College of Radiology41 defines patients at risk of NSF as those:
With advanced chronic kidney disease (eGFR < 30 mL/min/1.73 m2 not on dialysis)
On dialysis (any form)
With acute kidney injury.
In these patients, group I and III gadolinium-based contrast agents are contraindicated, with the caveat that there is insufficient real-life data to determine the risk of NSF from administration of group III agents. In patients at risk, if a gadolinium-enhanced MRI study is to be performed, a group II agent should be used. The lowest dose required to obtain the needed clinical information should be used, and it should generally not exceed the recommended single dose.
A summary of those recommendations with our comments and opinions is provided in Table 4.41
Key points from the ACR Manual on Contrast Media regarding prevention of nephrogenic systemic fibrosis in patients at risk
Gadolinium—the end of the story?
Although NSF has been basically eradicated since the guidelines were implemented, several cases of NSF have been reported in patients who never were exposed to gadolinium. In a review of biopsy-proven cases of NSF reported in 98 articles, 27 (8%) of 325 patients had no clear exposure to these agents,62 and in the review of 639 biopsy-proven cases discussed above, 14 (2%) did not.50 This suggests that gadolinium-based contrast agents are a major trigger for NSF, but they may not be the only one. Time will tell if indeed other triggers have yet to be discovered.
Additionally, in recent years, there have been data suggesting that gadolinium can deposit in the brain after repeated exposure to gadolinium-based contrast agents, even in patients with healthy kidneys.63 This finding was confirmed histologically64 and has led to the birth of a new term to describe it: gadolinium deposition disease.65 The significance of this brain deposition remains unknown, and to date, no adverse health effects have been uncovered. However, the FDA published a safety alert in 2015 indicating the active investigation of the risk and clinical significance of these gadolinium deposits. The recent position statement of the American College of Radiology also recognizes this phenomenon and states, “Until we fully understand the mechanisms involved and their clinical consequences, the safety and tissue deposition potential of all [gadolinium-based contrast agents] must be carefully evaluated.”66
It thus appears that we haven’t heard the last of gadolinium-based contrast agent-related disease. Additional research will be needed to understand the potential consequences of the use of these agents.
Footnotes
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.