ABSTRACT
Although uncommon, colovesical fistula creates significant morbidity, and many patients wait months to receive a correct diagnosis and treatment. Most cases are in older men who have diverticular disease, Crohn disease, cancer, or iatrogenic injury, and some of these associations may have occurred in the patient’s distant past and may not be immediately apparent. Since the incidence of diverticulitis in elderly patients is increasing and, in a separate trend, more patients are undergoing bladder instrumentation, we need to suspect this diagnosis when evaluating any patient with urinary tract infection, especially a man with prolonged symptoms refractory to conventional treatments.
Colovesical fistula is challenging to diagnose, as the signs and symptoms can resemble those of simple urinary tract infection.
There is currently no consensus on how best to diagnose colovesical fistula.
Urinalysis and urine culture offer no specific clues about anatomy and demonstrate only bacteriuria in more than 85% of cases.
The optimal treatment is surgery, but a medical approach is acceptable in patients who are too sick or frail to undergo surgery.
Colovesical fistula is by far the most common of the four types of enterovesical fistula (colovesical, rectovesical, ileovesical, and appendicovesical). First noticed by Rufus of Ephesus in CE 200, it was described officially by Cripps in 1888.1,2 It has been estimated to be responsible for 1 in 3,000 surgical admissions, typically occurring in men in their sixth or seventh decade.1,2
Colovesical fistula is a diagnostic challenge. Although it is an enteral disorder, the symptoms and signs mimic those of ordinary urinary tract infection.2–5 In addition, it is relatively rare, making large studies difficult, and thus there is no consensus on the best workup test or pathway.1 All of these factors contribute to delayed diagnosis and prolonged suffering.4–6
Here, we review the etiology, clinical presentation, diagnosis, and management of colonic fistula and propose a diagnostic approach.
DIVERTICULAR DISEASE CAUSES MOST CASES
The most common conditions that cause colovesical fistula in men are diverticular disease (responsible for 65% to 79% of cases in various series), malignancy (10%–20%), Crohn disease (5%–7%), and iatrogenic injury.1–3,7–9
Diverticular disease. The risk of developing a colovesical fistula in patients with diverticular disease is 1% to 4%.2,3,6,8 The mechanism is thought to be direct extension of a ruptured diverticulum or erosion of a peridiverticular abscess into the bladder. Peridiverticular phlegmon and abscesses are risk factors for future fistula formation.2,3,5
Malignancy. Advanced colon cancer and bladder cancer are the most common malignant causes of colovesical fistula. Less-common causes include urogenital malignancies and lymphomas.1,2 External-beam radiation to the bowel can induce endarteritis obliterans leading to necrosis and mucosal breakdown, which can contribute to fistula formation.
Crohn disease. About 2% of patients with Crohn disease develop colovesical fistula, most commonly iliovesical.1,4,5 Regional enteritis with transmural inflammation may result in adhesion of the inflamed section of the bowel to the bladder, followed by erosion with fistulization.1,4,5,6,10
Iatrogenic surgical injury is an uncommon cause of colovesical fistula but may be increasing in absolute numbers as more men undergo surgery in this part of the body. Colorectal, diverticular, and urologic surgery are some of the more common procedures associated with colovesical fistula.
Direct trauma such as a penetrating injury to the abdomen or pelvis is an uncommon cause of colovesical fistula.1
A MIMIC OF SIMPLE URINARY TRACT INFECTION
Although the cause of colovesical fistula is usually enteral, many patients present with urologic complaints.1,2,8,9 They can have long-term symptoms of recurrent urinary tract infection or asymptomatic bacteriuria, sometimes lasting months.10,11
The hallmark of any enterovesical fistula is Gouverneur syndrome, characterized by suprapubic pain, frequency, dysuria, and tenesmus.1,7 Symptoms can come from the gastrointestinal or urinary tract, but mostly from the latter.
Pneumaturia and fecaluria are pathognomonic and common.1,2,5,9
Abdominal pain is also common. It is not directly from the fistula, but is usually a late manifestation associated with Crohn disease with abdominal mass and abscess.1
Frequency, urgency, and suprapubic pain are present in almost all cases but are indistinguishable from symptoms of regular urinary tract infection.1,6
DIAGNOSIS IS A CHALLENGE
The diagnosis of colovesical fistula is clinical and a challenge for any clinician irrespective of training or specialty. There is no consensus on a diagnostic gold standard,1 and this disease is most commonly diagnosed through various tortuous, unusual, and sometimes unconventional clinical procedures.8 In most cases, the diagnosis is delayed or an afterthought.1,10
History
Normally, bacteria in the bladder get there by way of the urethra, and men, who have a longer urethra than women, are less vulnerable to urinary tract infection. Therefore, urinary tract infection in a male patient, especially recurrent infection, should raise suspicion for an underlying cause such as fistula. If urinary tract infection or bacteriuria recurs in any patient, a concerted effort is needed to identify an underlying cause. Important things to ask about in the history should include the following:
A history of instrumentation in the urogenital or gastrointestinal tract
A history of inflammatory bowel disease, external-beam radiation, or internal brachytherapy
How the patient recognized that he has urinary tract infection (eg, tenesmus, suprapubic pain)
Pneumaturia (Is your urine frothy? Are there bubbles in your urine stream?)
Fecaluria (Do you notice particles or cloudiness in your urine? Do you tend to push out cloudy urine during or after a bowel movement?).
Although a patient may not have paid attention to these symptoms before, asking may prompt him to look closer the next time he has urinary symptoms.
Physical examination
Common physical findings are fever, abdominal tenderness, and abdominal mass, although many patients have none of these.12 A more advanced examination should be done when this diagnosis is strongly suspected.
Laboratory testing
Some patients have anemia and leukocytosis.12 However, the laboratory approach usually relies on urinalysis, as blood test results tend to be within normal limits or nonspecific.5 Further, urinalysis and urine culture from midstream samples offer no specific clues, although they demonstrate significant bacteriuria in more than 85% of cases.2
The type of bacteria isolated may raise suspicion for various disease processes. Most urinary tract infections associated with colovesical fistula are caused by gram-negative bacteria, most often Escherichia coli. However, E coli is native to both the gastrointestinal and genitourinary tracts, and therefore if it is present in the urine it may have come from the gut—or not. Urinalysis by itself does not delineate the anatomy of the tract.1,2,12
Gram-positive bacteriuria, on the other hand, should always be evaluated critically. If Staphylococcus aureus (a gram-positive organism) is isolated in the urine, systemic bacteremia needs to be ruled out: in 2 series, the prevalence of bacteremia in patients with S aureus bacteriuria was 13%13 and 26.9%.14 If streptococci (another group of gram-positive organisms) are isolated in a man’s urine, an eroding malignancy and systemic bacteremia need to be ruled out.15 If the streptococci are enterococci, systemic bacteremia still needs to be ruled out, but the suspicion of colovesical fistula increases exponentially.
Special tests and imaging
If the clinical history and laboratory findings raise suspicion for colovesical fistula, numerous tests and imaging studies can be used to confirm it. However, their reliability varies.1,2
The poppy seed test involves feeding the patient 50 g of poppy seeds mixed with a beverage, yogurt, or something similar, and then examining the urine 48 hours later to see if these (relatively indigestible) seeds are coming out by that route. Kwon et al,16 in a series of 20 patients who ultimately underwent surgery and were found to have colovesical fistula, reported that this test was positive in all 20 patients (100%), whereas computed tomography yielded positive results in only 14 (70%).
Activated charcoal can also be ingested by mouth. If it is seen in the urine within 24 hours, this is considered diagnostic, with a reported sensitivity of 100%.2,5
Methylene blue test. Gynecologists who treat women with suspected vesicovaginal fistula often do a digital vaginal examination with a soft white gauze on the clinician’s gloved finger while a diluted solution of methylene blue in saline is infused into the bladder through a urinary catheter. If the gauze turns blue, there is a fistula.2,10 Similarly, gastroenterologists looking for colovesical fistula can infuse a tinted fluid such as methylene blue, with or without hydrogen peroxide, into the colon during sigmoidoscopy or colonoscopy. A blue tint in the urinary catheter indicates a fistula, and a diagnosis can be made.
However, Deshmukh et al17 found that methylene blue can be absorbed by the rectal mucosa and excreted by the kidneys and was therefore unreliable for confirming colovesical fistula. Indocyanine green can be used instead, with high specificity.2,8,17,18
Although these tests are inexpensive and easy to perform, they do not locate the fistula, and they may be unreliable.1,5,16
Cystoscopy has been regarded as the best diagnostic test for colovesical fistula. Woods et al,19 in a series of 53 patients with colovesical fistula, reported that they could directly visualize the fistula on cystoscopy in 24 (46%). However, they could see suggestive signs such as localized bullous edema with erythema or ulcer in 80% to 100% of the patients. Sou et al,18 using indocyanine green with cystoscopy, found the fistula in 11 (92%) of 12 patients.
Cystoscopy has thus been suggested as a first-line investigation.2,6,9,10,18,19 However, Golabek et al,1 in a review of 70 studies, found that cystoscopy yielded nonspecific findings, failing to identify colovesical fistula in 54% to 65% of cases.
Proctoscopy and colonoscopy have been suggested for every case of colovesical fistula. These procedures have a low detection rate, usually no more than 55%, but since 10% to 15% of cases of colovesical fistula are secondary to malignancy, endoscopy is still regarded as an essential part of the workup.5,10
Plain abdominal radiography is not helpful in diagnosing colovesical fistula, as the finding of air-fluid levels is not consistent with this diagnosis.1,9,19
Radiography with barium enema has a low diagnostic sensitivity of about 30%.1
Cystography similarly may show contrast outside of the bladder, marking a crescentic defect on the upper margin of the bladder representing a perivesical abscess. Like other plain imaging studies, it has a low detection rate of 20% to 30%.1,5,12
The Bourne test is radiographic evaluation of radiodense particles from a 24-hour urine collection after barium enema. It confirms colovesical fistula in up to 90% of cases. However, with advances in computed tomography, its role is decreasing.1,2,12,20
Computed tomography has become the test of choice for diagnosing colovesical fistula, recommended by the American College of Radiology as the first-line imaging test in suspected cases.5 It is widely available and noninvasive and provides explicit information not only about the location of the fistula but also about any surrounding inflammation, stricture, or malignancy, and is thus an aid to finding the underlying cause. It generates results quickly and has a diagnostic accuracy for colovesical fistula of up to 100%.5
The typical findings of colovesical fistula on computed tomography are air or contrast medium in the bladder and perivesical stranding with possible phlegmon or abscess nearby and adjacent thickened loops of bowel. However, other sources of air or contrast medium in the bladder that can present similarly and thus must be ruled out include recent urinary instrumentation or, in patients with diabetes, urinary tract infection with gas-forming organisms. A scan done with oral contrast that is then observed trickling into the bladder can help in both diagnosing a fistula and finding its location.1,2,5,11,12
Magnetic resonance imaging is a good alternative. It has high intrinsic soft-tissue resolution, which provides a better view of the fistula tract whether the communication is filled with air or fluid. It has sensitivity and specificity of up to 100%.5 Using intravenous gadolinium contrast improves the resolution and the accuracy of detecting bladder fistula. However, it is expensive and not available in every hospital, limiting its wider use.1,5,12
Currently, the European Association of Urology,21 American Association of Family Practice,22 and Infectious Disease Society of America23 do not recommend routinely performing cystoscopy or imaging in the diagnostic workup of recurrent urinary tract infection unless there is a high suspicion for renal calculi, outflow obstruction, interstitial cystitis, or urothelial cancer. When using imaging studies, the emphasis has been on minimizing radiation exposure.22 Documentation of the reason for the chosen imaging approach should include reasons beyond “recurrent UTI.”
Ultrasonography has therefore become the preferred imaging study in evaluating recurrent urinary tract infection. Golabek et al1 reported the usefulness of ultrasonography, and in some small series the detection rate of colovesical fistula has been up to 100%.1,24 With ultrasonography, the hallmark diagnostic sign is air in the bladder, although this is not specific. Applying abdominal pressure can enhance the yield by revealing the “beak sign” at the connection of the peristaltic bowel lumen with the urinary bladder.1,21–23 An innovative approach is to perform retrograde cystography and ultrasonography while the bladder is being filled with fluid. However, this has limited utility since most colovesical fistulas are unidirectional and flow from the colon into the bladder, as the bladder is more compliant than the colon.2,5,25
Our approach
In view of the considerations we have discussed, herein we propose our own approach.
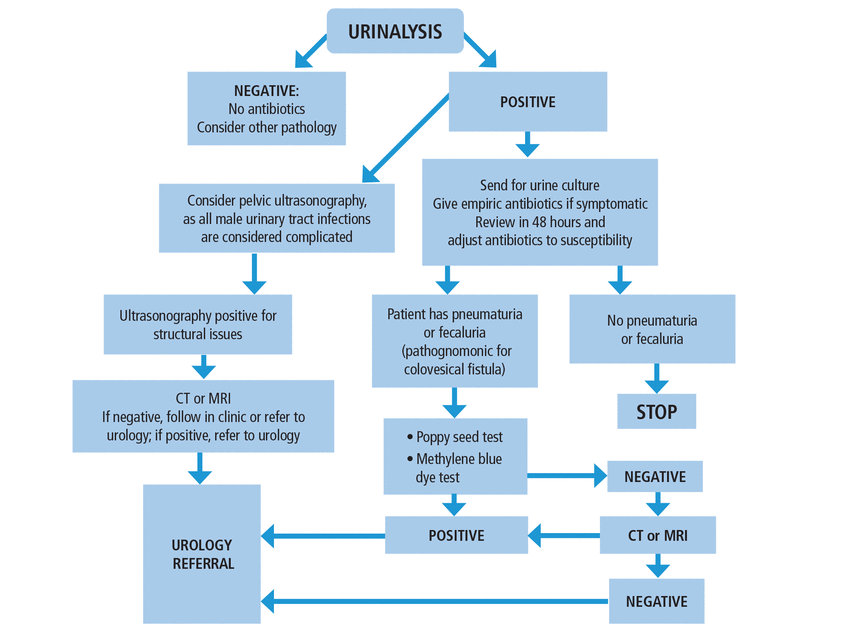
The first time a patient has a suspected urinary tract infection (Figure 1), urinalysis is the initial test. If the results of urinalysis are positive and the patient has typical symptoms of urinary tract infection and no pathognomonic symptoms, he can be treated empirically with antibiotics while awaiting culture results and considering ultrasonography. If the results are negative but pathognomonic symptoms of pneumaturia or fecaluria are present, we can consider a poppy seed test or methylene blue test. Computed tomography or magnetic resonance imaging can be used if these tests have negative results but the patient still has pathognomonic symptoms.
Our approach to male patients age 50 and older who have a first episode of suspected urinary tract infection.
CT = computed tomography; MRI = magnetic resonance imaging
For patients with recurrent urinary tract infection (Figure 2), urinalysis and ultrasonography can be considered initially. If the patient has positive results on ultrasonography or pathognomonic symptoms, then we consider a poppy seed test or methylene blue test, followed by computed tomography or magnetic resonance imaging of the abdomen and pelvis with contrast if the result is not definitive. Refer the patient to a urologist if colovesical fistula is definitively diagnosed or if the patient continues to have symptoms with an indeterminate diagnosis.
Our approach to male patients age 50 and older who have recurrent episodes of suspected urinary tract infection. CT = computed tomography; MRI = magnetic resonance imaging
SURGERY IS USUALLY REQUIRED
Although the best treatment for colovesical fistula can be debated, the definitive treatment is surgery.1,3,5,11,26 Endoscopic, open, and laparoscopic approaches have all been reported, and the choice depends on the underlying pathology, site of the bowel lesion, and the patient’s preoperative condition.1
Also open to discussion is whether to do the surgery all at once or over several stages. In a single-stage approach, the aim is to resect the primary lesion with anastomosis while also correcting the bladder defect. In a 2-stage approach, a diverting colostomy or Hartmann pouch is created after primary resection and anastomosis, which is then closed in a second procedure. Some perform a 3-stage operation to close the stoma.1,10 Lavery9 reported that most patients benefit from single-stage surgery.
Colovesical fistula can be managed conservatively in patients who are poor surgical candidates, those with minimal symptoms (particularly those with Crohn disease), or those who frankly refuse surgery.1,8,10,27 Golabek et al1 and Solkar et al8 reported that conservative therapy with a trial of bowel rest, total parenteral nutrition, antibiotics, steroids, immunomodulatory drugs, and urethral catheter drainage led to similar disease-specific mortality rates as with surgical treatment. However, others have reported significantly more deaths related to progression of malignant disease and septicemia: Garcea et al26 reviewed previous studies, which showed that up to 75% of patients with colovesical fistula who did not undergo surgery died of septic complications.
Historically, surgical management has been recommended to minimize risks of uremia or septicemia.8 However, surgery is not without complications. Solkar et al8 and Woods et al19 reported surgical morbidity rates of 4% and 45% and mortality rates of 0% and 30% in 2 small series. Technological advances and safer anesthesia and postoperative care have significantly reduced overall mortality rates in surgery of the colon in patients presenting with complications.3 Over time, surgery is essential to prevent recurrence and give the best overall benefit.1,26
Before surgery, endoscopy is recommended to rule out underlying malignancy.9
The outcome of colovesical fistula management is usually excellent, and recurrence after surgery is uncommon if the tissues are healthy and the underlying disease is not progressive.1
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Acknowledgment
We would like to thank Dwight D. Eisenhower VA Medical Center Department of Radiology for their assistance in preparing this report.
Footnotes
Disclaimer: The statements and opinions expressed in this review are those of the authors and do not represent an official position of any institution.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.