A diagnosis of amiodarone-induced thyrotoxicosis (AIT) should be considered in a patient started on amiodarone therapy who develops symptoms such as unexplained weight loss, diaphoresis, tremor, palpitations, or anxiety. Prompt diagnosis with serologic testing of thyroid-stimulating hormone (TSH), free thyroxine (T4), and triiodothyronine (T3) levels is crucial.
AIT can be classified as type 1 (AIT-1) or type 2 (AIT-2). AIT-1 occurs in the setting of underlying thyroid disease (often undiagnosed) while AIT-2 is due to amiodarone toxicity without underlying glandular pathology. Differentiation between these forms of AIT requires radioactive iodine uptake and Doppler ultrasonography.1,2
Radioactive iodine uptake studies typically show normal or increased uptake in AIT-1, whereas AIT-2 typically has decreased uptake. In fact, uptake in AIT-1 may be decreased due to high iodine load in amiodarone competing with uptake by the thyroid gland. Due to related concerns regarding inadequate accuracy of radioactive iodine uptake studies in distinguishing between AIT-1 and AIT-2, technetium Tc 99m methoxyisobutylisonitrile thyroid scintigraphy has emerged as a more sensitive and accurate diagnostic imaging modality. Increased uptake and retention of technetium Tc 99m methoxyisobutylisonitrile in thyroid tissue is seen in AIT-1, whereas low uptake is seen in AIT-2.1,2
Doppler ultrasonography shows increased vascularity and blood-flow velocity in AIT-1, whereas these findings are absent in AIT-2. AIT-1 is best treated with antithyroid drugs and potassium perchlorate. AIT-2 is typically self-limited and can be treated with steroids. Mixed forms with imaging and clinical findings of both AIT-1 and AIT-2 are also seen and are difficult to diagnose. Management of these forms involves a combination of thionamides and steroids. Thyroidectomy may be done in cases of hemodynamic instability or clinical worsening.
PHYSIOLOGY OF AMIODARONE-INDUCED THYROTOXICOSIS
Amiodarone is an iodine-rich antiarrhythmic drug3 that can cause thyrotoxicity through several mechanisms. Iodine is required for thyroid hormone synthesis. Due to its structural similarity to the hormone T3, amiodarone can mimic the actions of T3 and lead to thyrotoxicosis.4
Amiodarone is a benzofuran derivative with 2 iodine atoms that are released into systemic circulation. The recommended daily intake of iodine is about 0.2 mg. However, a maintenance dosage of amiodarone at 200 mg/day provides 7.5 mg of organic iodide per day. The accumulation of systemic iodine is also known to precipitate thyrotoxicosis. Amiodarone has a half-life of 100 days, with a large volume of distribution, which further enhances its toxicity and predisposes to drug withdrawal.5
Normally, autoregulatory mechanisms prevent large quantities of iodine from accumulating and leading to excessive production of thyroid hormones. The Wolff-Chaikoff effect is a protective phenomenon wherein elevated plasma iodine concentrations temporarily halt the synthesis of thyroid hormones.3 The escape from this protective effect leads to the Jod-Basedow effect, wherein thyroid gland hyperactivation or autonomous production by thyroid nodules occurs in the absence of negative feedback to the pituitary gland.3
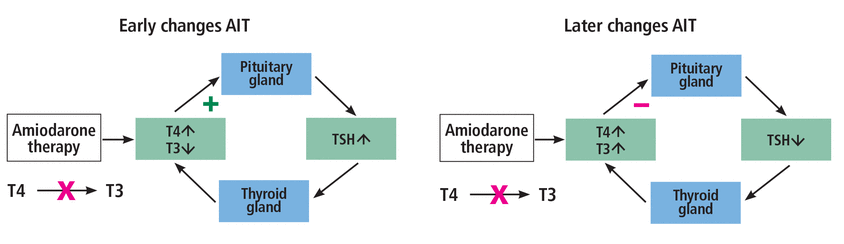
Amiodarone-induced thyrotoxicosis can be confirmed by low TSH, high T4, and high-normal or high T3. Additionally, patients may be asymptomatic or may exhibit signs of overt thyrotoxicosis, including palpitations, tremors, diaphoresis, and weight loss.4 AIT-1 is caused by the excessive iodide content of amiodarone leading to increased thyroid hormone synthesis in the absence of TSH stimulation. The new steady state in AIT-2 consists of elevated T4, low T3, and normal TSH levels (Figure 1). AIT-2, also known as destructive thyroiditis, is due to the toxic effects of amiodarone on thyroid follicles, leading to the release of stored thyroid hormones.
Early and late stages of amiodarone-induced thyrotoxicosis (AIT). (A) In early AIT, amiodarone blocks the conversion of thyroxine (T4) to triiodothyronine (T3), leading to increased levels of T4 and decreased levels of T3. Through feedback, the pituitary gland is stimulated to produce more thyroid-stimulating hormone (TSH), which promotes thyroid hormone production by the thyroid gland. (B) In late AIT, after the increased TSH production and stimulation of the thyroid gland, T3 and T4 levels both become elevated. Through negative feedback on the pituitary gland, less TSH is secreted.
TYPE 1 AMIODARONE-INDUCED THYROTOXICOSIS
AIT-1 is a form of true hyperthyroidism driven by autonomous thyroid hormone production in the presence of iodine overload.4 It occurs in patients with concomitant thyroid disorders, such as nodular goiters or Graves disease.6
Diagnosis of AIT-1 typically includes radioactive iodine uptake studies that show normal or increased uptake. Additionally, color-flow Doppler ultrasonography helps distinguish AIT-1 from AIT-2. Increased vascularity and blood-flow velocity on color flow Doppler suggest AIT-16 (Table 1). Treatment of AIT-1 involves antithyroid agents like methimazole and propylthiouracil, which inhibit iodide uptake by the gland and by new hormone synthesis. In addition, propylthiouracil inhibits conversion of T4 to T3. The addition of potassium perchlorate can increase the response to the aforementioned thionamides and further inhibit iodine uptake.4 Medical therapy is usually required for several weeks to achieve a euthyroid state. For medically refractory cases of AIT-1, thyroidectomy can be considered.4
Features of type 1, type 2, and mixed amiodarone-induced thyrotoxicosis
TYPE 2 AMIODARONE-INDUCED THYROTOXICOSIS
AIT-2, a form of drug-induced destructive thyroiditis, occurs due to true toxicity from amiodarone rather than underlying thyroid disease as in AIT-1. Due to the overlap in presentation between both types of AIT, radioactive iodine uptake studies and color-flow Doppler can aid in the diagnosis. Radioactive iodine uptake is typically less than 3% in AIT-2, and Doppler ultrasonography usually has no hypervascularity pattern or blood-flow velocity.6
AIT-2, unlike AIT-1, is commonly self-limited and is best managed with corticosteroid therapy. Treatment with weight-based dosing of prednisone (0.5–0.7 mg/kg/day) for 1 to 3 months has been shown to be effective very early on, and around 50% of patients with AIT-2 have complete resolution of disease in 4 weeks6 (Table 1).
MIXED AMIODARONE-INDUCED THYROTOXICOSIS
In some cases, patients may have a mixed form of AIT, ie, AIT-1 and AIT-2, physiologically driven by pathological mechanisms that also drive AIT-1 and AIT-2. As such, the mixed form can present phenotypically as either AIT-1 or AIT-2, making diagnosis a challenge.
Management typically involves thionamides and steroids. Potassium perchlorate may also be used but is not readily available in the United States. Early initiation of therapy is crucial, as studies have shown increased rates of mortality in patients with mixed AIT, especially in patients with underlying cardiac dysfunction.7
CONTINUATION OF AMIODARONE AFTER THYROTOXICOSIS
The decision to continue or discontinue amiodarone after resolution of AIT is controversial. Ultimately, this decision needs to be made on an individual basis. Some case reports suggest that continuation of amiodarone is acceptable in patients suffering from life-threatening arrhythmias or arrhythmias that have been resistant to other medical therapies.4 If amiodarone therapy is maintained, it is important to note the risk for recurrent thyrotoxicosis and inform patients of this risk during shared decision-making. Pacemakers or implantable cardioverter defibrillators may also be utilized along with other medical methods of arrhythmia control.
THE BOTTOM LINE
Differentiating between AIT-1 and AIT-2 requires a combination of serologic testing, color Doppler ultrasonography, and radioisotope studies.
Treatment of AIT-1 requires antithyroid agents like methimazole and propylthiouracil whereas AIT-2 is typically self-limited and treated with steroids.
Thyroidectomy may be performed in both types of AIT if the patient is hemodynamically unstable.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.