As of may 11, 2024, only 15% of US children and 22% of US adults had received the updated 2023–2024 COVID-19 vaccine (42% of those 75 and older). Similarly, only 54% of children, 48% of adults, and 78% of adults age 75 and older had received the updated influenza vaccine, while 23% of adults 60 or older had received a respiratory syncytial virus (RSV) shot.1 In contrast, immunization rates for standard childhood vaccinations remain in the range of 90% for those born in 2019 and 2020.2
These numbers are below the targets, especially for COVID-19 vaccination. The 3 COVID-19 vaccines available and authorized for use in the United States are safe and effective and have highly favorable risk-benefit profiles. They are relatively easy to obtain, and the Centers for Disease Control and Prevention has issued clear recommendations for using them. And so it is frustrating for many healthcare professionals to repeatedly see patients who refuse to be vaccinated.
Below, we review the history of vaccine hesitancy, what we do and do not know about the currently available COVID-19 vaccines, and ways for clinicians to help patients decide whether to be vaccinated against COVID-19.
VACCINE HESITANCY IS NOT NEW
Vaccine hesitancy did not start with the COVID-19 pandemic.3 Skepticism regarding the value of vaccination dates to the ancient practice of variolation (intradermal insertion of material from smallpox blisters, which minimized the impact of any subsequent natural smallpox infection), which became popular in Europe and the American colonies in the 18th century. For example, in Boston in 1721, Dr. Zabdiel Boylston began performing variolation (which he learned from an enslaved African person) in an attempt to stem an epidemic of smallpox. He was supported in this practice by royal governor Samuel Shute and theologian Cotton Mather—and opposed by local patriots that included a young printer’s apprentice named Benjamin Franklin. Dr. Boylston had to go into hiding, and Reverend Mather’s house was firebombed.4 Things did not change much in the 19th century when variolation was replaced with cowpox (vaccinia) as the first vaccine (Figure 1).
“The Cow Pock—or—the Wonderful Effects of the New Inoculation” by cartoon satirist James Gillray, June 12, 1802. Portrays a scene from the Smallpox and Inoculation Hospital at St. Pancras of people taking the shape of cows after being inoculated with vaccinia by Edward Jenner.
Reproduced from Library of Congress. http://hdl.loc.gov/loc.pnp/ds.14062.
The 1853 British Compulsory Vaccination Act, requiring smallpox vaccination for infants, was met with fierce and at times violent resistance by the working class, who saw it as the latest oppressive move by the ruling class to exert control over their bodies. This resistance was only enhanced by the fact that those who refused to have their children vaccinated were severely fined or thrown into jail under harsh conditions.3,5
Similarly, antivaccination sentiments in the United States at the end of the 19th century were also initially a reaction to mandatory vaccination laws. Of note, the Supreme Court at that time ruled that such laws were constitutional if they were necessary to ensure public safety.6 The clear decreases in morbidity and mortality from smallpox and polio following large-scale vaccination campaigns led to a general acceptance of the safety and efficacy of vaccines.
A number of events over the past 75 years has led to public concerns about vaccine safety and efficacy. As is often the case, the full story took longer to emerge.3 Among these events were the following:
Inadequate inactivation of polio vaccine, leading to tens of thousands of cases of polio and 10 deaths (This happened in 1955, and this vaccine is no longer used.)
Contamination of polio vaccines with SV40 virus (But no clinical consequences of SV40 contamination were found.)
A 1-in-100,000-person increase in cases of Guillain-Barré syndrome during the 1976 influenza vaccination campaign (The risk of Guillain-Barré syndrome following influenza vaccination is currently closer to 1 excess case in 1 million, which is lower than the risk following influenza infection.)
Neurologic complications from diphtheria-tetanus-pertussis vaccine (The risks were determined to be extremely low, and a decrease in vaccination in the United Kingdom led to a significant outbreak of pertussis.)
Claims of autism in association with the measles-mumps-rubella vaccine. (The article reporting this association was found to be flawed and retracted by the publisher [The Lancet]. Financial ties were revealed between the primary author of that article and attorneys pursuing legal action against vaccine manufacturers.7)
Along with selfie and CRISPR, the term vaccine hesitancy first appeared in the English language in 2002 (www.merriam-webster.com/time-traveler/2001). It was initially included in the Oxford English Dictionary in 2006 and is defined there as hesitancy, reluctance, or refusal to have oneself or one’s children vaccinated against an infectious disease or diseases. Vaccine resistance describes an extreme form in which people are not merely unsure but are actually opposed to vaccination. Complacency, inconvenience in accessing vaccines, and lack of confidence are key factors underlying vaccine hesitancy.
VACCINE HESITANCY AS A THREAT TO HEALTH
Vaccination has had a substantial positive impact on both individual health and public health, but its gains are compromised by vaccine hesitancy. In 2019, the World Health Organization identified vaccine hesitancy as 1 of the top 10 threats to global health.8 They noted that vaccination currently prevents 2 to 3 million deaths a year and that an additional 1.5 million deaths could be prevented if vaccination rates were higher.
Successes of vaccination campaigns
At the level of individual health, vaccines have decreased morbidity and mortality from a variety of infectious diseases both by reducing the risk of new infection and by minimizing the impact of infection in individuals who become infected despite vaccination. Notable successes include vaccines against measles, diphtheria, varicella zoster (which causes chicken pox and shingles), and human papillomavirus (which causes cervical dysplasia and cancer).
As for public health, vaccinations can decrease the spread of infection and the burden on the healthcare system. Vaccination campaigns have eradicated smallpox, are closing in on eradicating polio, and have “eliminated” measles in the United States, at least for the time being. (In this context, “elimination” means no endemic measles transmission for at least 1 year in the presence of a well-performing surveillance system.)
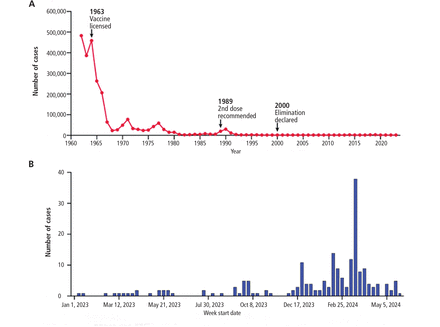
Measles deserves special mention. While vaccination rates for measles-mumps-rubella and polio remain high overall, there are pockets where decreasing rates of vaccination have led to recent outbreaks of measles. Worldwide cases of measles surged by 30% in 2019, which was attributed, at least in part, to vaccine hesitancy.8 In the United States, the “eliminated” status of measles is at risk, with 159 cases reported in the first 6 months of 2024 (Figure 2).9 At the same time, the vaccination rate among kindergartners has declined, from 95.2% during the 2019–2020 school year to 93.1% in the 2022–2023 school year.9 Recent trends—an increase in the number of cases and declines in immunization rates—indicate that gains can be vulnerable and depend upon ongoing public health efforts to maintain high rates of acceptance of the measles-mumps-rubella vaccine.
Measles cases in the United States (A) 1962 to 2023 and (B) January 2023 to March 2024.
Adapted from reference 9.
Varicella zoster. In addition to reducing the incidence of childhood infectious diseases, several vaccines also prevent some of the long-term consequences of infections. For example, the childhood varicella-zoster vaccine decreases the risk of shingles later in life, and the human papillomavirus vaccine given at ages 9 to 26 years decreases the risk of cervical dysplasia and cancer. The 2-dose varicella-zoster childhood vaccine in the United States (typically given in combination with measles-mumps-rubella) has led to approximately a 90% decline in the incidence of diagnosed infections, hospitalizations, and death due to varicella zoster.10 And in multiple studies, people who were vaccinated in childhood had about a 50% lower incidence of shingles later in life.10
Human papillomavirus. Even more striking, in cancer prevention, women who received the quadrivalent human papillomavirus vaccine before age 17 were approximately 90% less likely to develop invasive cervical cancer later in life, and those who received it between ages 17 and 30 were about 50% less likely.11 A Cochrane review of 26 randomized controlled trials with 73,428 participants found that women age 15 to 25 years, negative for any high-risk human papillomavirus subtype at study entry, who received the vaccine had a 63% lower risk of precancerous lesions, with a number needed to vaccinate of 55.12
COVID-19 vaccines are effective, but degree of efficacy is hard to determine in 2024
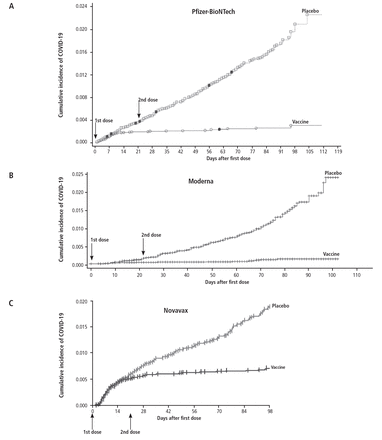
The 3 COVID-19 vaccines available in the United States—the Pfizer-BioNTech (Comirnaty) and Moderna (Spikevax) mRNA vaccines and the Novavax (NVX-CoV2373) adjuvanted protein vaccine—have also shown similar impressive degrees of efficacy (Figure 3).13–19 In the pivotal phase 3 studies that led to the emergency use authorizations for these vaccines, they decreased the incidence of severe disease by 90% to 100% (Table 1).15–17
Efficacy and safety of COVID-19 vaccines
Unfortunately, it is difficult to precisely ascertain their current efficacy, and in turn to provide precise information to the public about their efficacy at this time. This is because the circulating variant is different (Alpha vs Omicron KP.3) and the preexisting level of host immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; either from prior infection or vaccination) in the general population is different as well.
While some vaccines confer lifelong protection (particularly the live virus vaccines such as vaccinia), the COVID-19 vaccines probably do not, and periodic booster immunizations are recommended. Currently available data suggest that serum antibody levels decline faster with the mRNA vaccines than with the protein vaccines.20 However, for most vaccines, memory B cells and T cells (which mount an immune response on reexposure) may persist for considerably longer than plasma antibodies. This is an area of current study.
One thing we can say with assurance, however, is rates of serious adverse events are very low with these vaccines, with similar rates in the placebo and vaccine groups in the pivotal studies (Table 1).15–17
Complicating any meaningful discussion about the current efficacy of the COVID-19 vaccines, the estimates, other than those derived from the pivotal phase 3 studies, vary widely in both the scientific and lay literature. Some of these differences are due to different definitions of efficacy being used, eg, rates of overall infection vs rates of symptomatic infection vs rates of serious illness or death.
Other differences derive from the different methodologies used. These range from the gold standard of a randomized, double-blind controlled trial to the more convenient use of observational cohorts. These latter studies are often referred to as “real-world evidence”21,22 and typically compare outcomes between people who have or have not been vaccinated. While they control for a variety of known and measured variables as best they can, they remain confounded by unrecognized variables. For example, people who elect to be vaccinated and get booster shots probably differ in ways we do not measure (such as degree of risk-taking behaviors) from those who do not. Those differences might influence the relative risk of exposure to SARS-CoV-2—for example, people who are opposed to social distancing and masking are more likely to be opposed to vaccination.23
Thus, it is hard to draw a firm conclusion about the current level of efficacy of these vaccines. It is fair to say that they are effective, but the magnitude of that efficacy is not clear.
Vaccination prevents severe COVID-19
The COVID-19 vaccines appear to be most effective in preventing severe disease and death and least effective in preventing infection itself. In other words, an infection in someone who is vaccinated does not mean that the vaccine does not work; the COVID-19 vaccines, like most others, may not prevent infection but do greatly decrease the impact of infection. Data from the Omicron period suggest that vaccination is associated with a 62% decrease in hospitalization and 69% decrease in critical illness during the first 2 months following vaccination, dropping to a 24% decrease in hospitalization and a 50% decrease in critical illness during months 4 to 6.24,25
Given the strong, consistent data indicating that the risk of vaccination is low (discussed below), one can conclude that the risk-benefit ratio remains strongly in favor of vaccination. Thus, it is important for the clinician to provide context as to the nature of that benefit, namely protection from severe disease, when making such a statement to a prospective vaccine recipient. It is also worth noting that efficacy decreases with time after the last shot, making a strong case for getting periodic boosters.
To be clear, the discussion on whether to be vaccinated when the vaccines were first available, when there had not yet been widespread exposure to SARS-CoV-2 and the circulating variants were more virulent, was much less nuanced than the situation today. At that time, the data from the randomized controlled trials were current and the mRNA vaccines were shown to be safe and effective—especially from the perspective of preventing death. Appreciating this difference will be critical to an effective response to the next pandemic.
DISCUSS THE PROS AND CONS, BUT DON’T ARGUE WITH PATIENTS
Because we live in an environment of conflicting information, an important key to discussing the risks and benefits of any indicated vaccine with patients is to avoid getting into an adversarial relationship. To start, acknowledge that the patient has the final word on what they elect to do and that your job is to provide them with reliable information on which they can base their decision. Indicate you will provide a clear recommendation based on the available information while at the same time acknowledging that there are still some unknowns.
While suspicion of physicians and hospitals in general is widespread, individuals typically have high confidence in their own clinician, especially if they have a long-standing relationship. A survey commissioned by the American Board of Internal Medicine Foundation carried out from December 2020 through January 2021 concluded that trust in individual clinicians is greater than trust in the healthcare system as a whole;26 however, trust in physicians decreased during the COVID-19 pandemic and needs to be rebuilt.26,27
In discussions about vaccine safety and efficacy, point out that one cannot rely on social media, which typically have no filters or peer review on what is posted. As a consequence, such postings may not be based on evidence or data and may instead be based on politics and beliefs. For example, in the survey cited above, 78% of Democrats said they had confidence in their doctor to administer a COVID-19 vaccine compared with 51% of Republicans.26
While the survey did not explore the reasons for these differences, a plausible explanation may be the sources of their information via commercial and social media. Psychologist Dan Ariely of Duke University has coined the term “funnel of misbelief” to describe the way in which rational people may end up with very different views of the world based on their emotions, degree of stress, cognitive biases, personality, and exposure to different types of social forces.28 When we don’t understand what is going on around us (eg, a COVID pandemic), there is a deep psychological need to come up with some narrative, real or imaginary, to explain things.
It is often stated that one is entitled to their own opinions, but not their own facts. While the facts regarding the safety and efficacy of many vaccines, including the COVID-19 vaccines, are clear, the way they are interpreted through a political lens can be confusing. It is the responsibility of the clinician to help the patient identify the facts so that they may reach an informed decision. An approach being studied is the 4-step technique of “empathetic refutation,”29 in which the clinician:
Elicits concern (asking patients to share their thoughts to uncover what they perceive as the underlying facts)
Affirms whatever truths are contained in their thoughts
Offers a tailored refutation of any misconceptions with facts
Provides additional facts in support of vaccination.
It is important to avoid value judgments and instead to listen and support without becoming argumentative. The patient’s perspective on the topic may be more related to the degree of emotion with which they approach the issue rather than stemming from disagreement regarding the facts.
In her book Thinking in Bets,30 poker champion Annie Duke notes that people may most easily accept the first thing they hear to be true and that it may take some time to move to a different position. She goes on to note that it is important to communicate one’s own degree of uncertainty when discussing controversial issues and frame a discussion moving from acknowledgement of uncertainty to identifying areas of agreement (for example, COVID can cause severe illnesses and death) and from there discussing ways to avoid a bad outcome. In other words, spend time focusing on and agreeing on the problem before moving to potential solutions.
Egregious misinformation has arisen from false claims regarding the danger of vaccines through inaccurate interpretations of the incidence of adverse events that occur following vaccination. An adverse event is any undesirable experience that occurs after a medical product is used in a patient. In this regard, it is important to distinguish between an adverse event that is due to a vaccine vs an adverse event not due to a vaccine occurring in a person who coincidentally received a vaccine.
The cleanest data on adverse events of vaccines come from the randomized placebo-controlled trials that are done early in the testing of a new vaccine (Table 1).15–17 Additional data come from postauthorization and postlicensure reporting to the Vaccine Adverse Event Reporting System (VAERS). The randomized controlled trials allow a clear distinction between events due to the vaccine (seen more frequently in the vaccine than in the placebo group) and those that would have occurred regardless of vaccination (seen at the same frequency in both groups). While not as robust, VAERS data can be particularly valuable in helping to spot a rare vaccine-related adverse event by comparing the incidence of the event in vaccinated individuals vs in the general population.
What are the risks from the COVID-19 vaccines?
After close to 8 million doses of the Janssen (Johnson & Johnson) Ad26 COVID-19 vaccine had been given in the United States, 17 cases of the thrombosis with thrombocytopenia syndrome were reported to the VAERS.31 This was an approximately 15-fold relative risk, although a small absolute risk, and appeared to be focused in women 18 to 49 years of age. In response, the Centers for Disease Control and Prevention modified its recommendations for use of the Ad26 platform vaccine,32 and the observation likely played a role in the June 2023 revocation of the US emergency use authorization of this vaccine following a request from Janssen. This example can be used to illustrate some of the steps taken in the United States to monitor even rare vaccine risks and the subsequent actions taken when a new risk is identified.
Some claim that all reported adverse events in vaccine recipients are due to the vaccine. This can be confusing to the public. As noted above, it can be easy to conflate adverse events due to a vaccine with adverse events not due to a vaccine in someone who has received a vaccine. For example, every day most people drink water, but not everyone who got sick on a given day became ill from the water they drank; in some instances that might be true, in other instances not. As noted above, the best way to determine the impact of an intervention is in a randomized placebo-controlled trial, the exact type of trial that led to the authorizations and licensures of the current COVID-19 vaccines.
It is true that these vaccines were developed in record time and initially provided on the basis of emergency use authorization. However, it is important to point out that the study designs, with approximately 30,000 individuals per study and subsequent follow-up for a minimum of 2 years, that led to their formal licensure were comparable to designs of studies done for other licensed vaccines.
For the Moderna RNA vaccine, the frequency of serious adverse events was similar in the placebo and vaccine groups (1.4% vs 1.6%).16 For the Pfizer-BioNTech vaccine, the frequency of serious adverse events after 1 dose was 0.5% for the placebo group and 0.6% for the vaccine group.15 For the Novavax vaccine,17 the frequency of any serious treatment-emergent adverse event was 1.0% for the placebo group and 0.9% for the vaccine group (Table 1). While one cannot use these numbers to compare the vaccines to each other, owing to differences in the precise definitions used in the different studies, it is clear that the incidence of serious events was comparable between the placebo and vaccine groups in each study.
As expected, less-serious events, especially local reactions, were more frequent in the vaccine groups than in the placebo groups of the studies. The rates of total local adverse events after the second shot in the placebo and vaccine groups, respectively, were 43% vs 92% for the Moderna vaccine,16 12% vs 78% for the Pfizer-BioNTech vaccine,15 and 22% vs 79% for the Novavax vaccine.17
An evidence-based review of the adverse effects of COVID-19 vaccination and intramuscular vaccine administration conducted by the independent National Academies of Science, Engineering and Medicine,33 released in 2024, concluded that overall the most common side effects associated with COVID-19 vaccines were similar to those of other vaccines, ie, flu-like syndromes and local reactions at the injection sites. The review, however, did note convincing evidence of a causal relationship between the mRNA vaccines and myocarditis. The frequency of these events was too low to be detected in the randomized controlled trials, with the evidence of the association coming from the observational cohort studies and reporting to VAERS—again demonstrating the importance of the different ways safety signals are pursued. Overall, this risk was on the order of 7 in 100,000 in vaccine recipients (compared with a pre-COVID rate of 1 in 100,000), more common in white males ages 16 through 30, more common with the second dose, and rarely seen in individuals over 50. Of note, these rates are considerably lower than the rate of myocarditis following COVID-19 (150 in 100,000), a rate that is at least halved with prior vaccination.34,35
The National Academies Review Committee also concluded that there was no relationship between the mRNA vaccines and thrombosis with thrombocytopenia syndrome, infertility, Guillain-Barré syndrome, Bell palsy, or myocardial infarction.33 In contrast, they did report that there was sufficient evidence to conclude that there is a causal relationship between the Ad26 and ChAd platform COVID-19 vaccines and the thrombosis with thrombocytopenia syndrome and the Guillain-Barré syndrome. Of note, these latter 2 vaccines are not available in the United States.
Thus, while the COVID-19 vaccines available in the United States have some risks, severe adverse effects due to the vaccines are rare and the risks are greatly outweighed by the benefits. In everyday life one takes risks in order to derive benefits.
A RELATIONSHIP BUILT ON TRUST
In discussing vaccines in general and COVID-19 vaccines in particular, it is important to empower patients to be their own advocate while helping them sort through the information, emphasizing what we know and where uncertainty remains. To use an analogy, patients typically trust that high blood pressure is bad and should be managed—including with drugs that have a number of side effects. The medical profession needs to work to develop a similar level of trust in the science behind the licensure of vaccines. For COVID-19 vaccines, it is important for the clinician to provide their patients with an objective view of our current understanding of the safety and efficacy of these vaccines and to employ shared decision-making to maintain a relationship built on trust.
Vaccines have been some of the most effective strategies we have to decrease the morbidity and mortality of many infectious diseases, and they need to remain front and center in dealing with today’s infectious disease threats as well as those of tomorrow. By neither overstating nor understating their safety and efficacy we may be able to optimize their value today and in the future.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.