A man in his 30s presented to his primary care clinician for new irritability, anxiety, and insomnia, without suicidal ideation. Symptoms began after an increase in job-related stress and the anniversary of his mother’s death.
His medical history included obesity, depression, attention-deficit hyperactivity disorder, irritable bowel syndrome, and erectile dysfunction. He was a lifelong nonsmoker and did not use illicit drugs. Current medications were vitamin D and sildenafil. In addition, he had been treated with chemotherapy (unknown regimen; no radiation) at age 3 years for acute lymphoblastic leukemia.
INITIAL EVALUATION AND MANAGEMENT
At the primary care visit, his temperature was 36.7°C (98°F), heart rate 90 beats per minute, and blood pressure 119/81 mm Hg. Physical examination was unremarkable, with normal heart, lung, abdomen, skin, and neurologic evaluations, including absence of murmur, tremor, or edema.
The differential diagnosis for generalized anxiety is broad and includes cardiac disorders (eg, arrythmia or heart failure), endocrine disorders (eg, hyperthyroidism or hypoglycemia), and psychiatric disorders (eg, anxiety).1 His primary care clinician favored anxiety and recommended behavioral counseling along with sertraline 50 mg daily. Given his acute lymphoblastic leukemia history and the development of new symptoms (by their mid-40s, approximately 95% of those who survived childhood cancer will have a health problem related to the cancer diagnosis or treatment2), laboratory tests were also ordered.
Initial laboratory tests showed no evidence of anemia, electrolyte disturbance, hypoglycemia, kidney failure, or liver failure (Table 1) but revealed a thyroid-stimulating hormone (TSH) level of 0.025 mIU/L (reference range 0.350–4.920 mIU/L). Repeat thyroid function tests 1 month later showed a similarly low TSH level of 0.019 mIU/L but a normal free thyroxine (T4) level of 1.01 ng/dL (0.70–1.48 ng/dL). Due to persistently low TSH, he was referred to endocrinology.
Initial laboratory results
ENDOCRINOLOGY EVALUATION
Two weeks later at the endocrinology visit, he reported worsening anxiety despite ongoing use of sertraline 50 mg daily. He denied heat intolerance, tremors, eye pain, vision changes, or neck discomfort. His weight was stable and had not varied by more than 3 pounds during the previous 6 months.
He had no recent history of iodinated contrast or infections and was not taking iodine supplements. There was no family history of thyroid disease, but his sister was thought to have ulcerative colitis. Although childhood cancer survivors, like this patient, have higher rates of hyperthyroidism, it is typically linked to radiation treatment rather than to chemotherapy or the cancer diagnosis.3 Interestingly, he had been in Japan near the tsunami-related nuclear power plant disaster in 2011.
Physical examination revealed mild thyromegaly without a discrete nodule, tremors, proptosis, or lid lag. His temperature was 36.1°C (97°F), heart rate 74 beats per minute, and blood pressure 122/70 mm Hg. Heart sounds were regular without murmurs. Breathing was nonlabored with no adventitious sounds. No rash, edema, ataxia, or other abnormality was noted.
Neck ultrasonography was ordered because of the goiter, and results of laboratory testing that day showed the following:
TSH 0.021 mIU/L
Free T4 1.02 ng/dL
Free triiodothyronine (T3) 3.53 pg/mL (1.71–3.71 pg/mL)
Thyroid-stimulating immunoglobulin 0.42 IU/L (≤ 0.54 IU/L).
DIFFERENTIAL DIAGNOSIS
1. Which of the following is the most likely diagnosis based on results of his thyroid function tests?
Central hypothyroidism
Euthyroid sick syndrome (nonthyroidal illness)
Drug-mediated suppression of TSH
Subclinical hyperthyroidism due to Graves disease or autonomous nodules
The differential diagnosis of low TSH includes causes of overt or subclinical hyperthyroidism, thyroiditis, hypothalamic or pituitary disease, euthyroid sick syndrome, and drug-mediated suppression (Table 2). A thorough history and physical examination can narrow the differential.
Differential diagnosis of low thyroid-stimulating hormone level
The patient’s potential radiation exposure while he was in Japan could have increased the risk of a thyroid nodule, and a goiter was felt on examination. He had no neck discomfort, iodine exposure, or recent infection, making thyroiditis less likely. His free T4 level was in the mid-normal range, which ruled out hypothalamic or pituitary disease because a lower T4 level is expected in central hypothyroidism. He had no other acute or sub-acute illness, so euthyroid sick syndrome, which results in a transient reduction in TSH, was unlikely. He also was not taking medications that would affect thyroid function. While the underlying cause was unknown, the most correct endocrine diagnosis at this point was subclinical hyperthyroidism.
Subclinical hyperthyroidism is defined as low or undetectable TSH with normal T3 and free T4 levels.4 Overt hyperthyroidism is defined as TSH below the lower limit of normal with an elevated level of T3 (either free or total), free T4, or both. Obtaining a T3 level is important because some patients, especially those with Graves disease, present with T3 thyrotoxicosis with low TSH, normal free T4, and elevated T3 levels.4 Subclinical hyperthyroidism typically has fewer or no symptoms compared with overt hyperthyroidism (Table 3).4
Hyperthyroidism signs and symptoms
Overt hyperthyroidism requires treatment; subclinical hyperthyroidism requires treatment only in select patients. A TSH level less than 0.10 mIU/L has been associated with an increased risk of atrial fibrillation, decreased bone density in postmenopausal women, and a higher likelihood of progression to overt hyperthyroidism.5 The American Thyroid Association recommends considering TSH level, age, comorbidities, and risks when deciding whether to treat subclinical hyperthyroidism (Table 4).6
Summary of American Thyroid Association recommendations for treatment of subclinical hyperthyroidism
CASE CONTINUED: FINDINGS ON IMAGING, SUBSEQUENT FINE-NEEDLE ASPIRATION
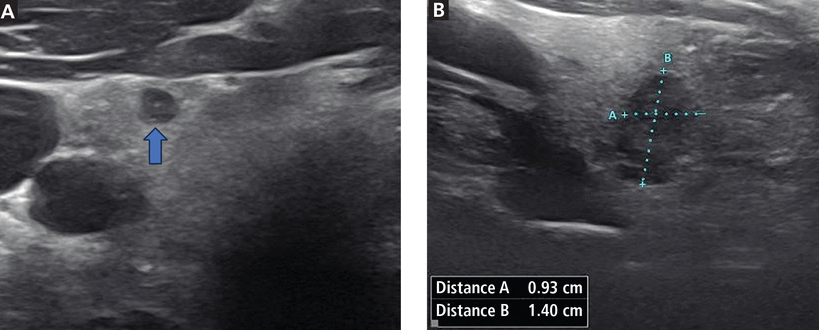
A week later, neck ultrasonography showed a mildly enlarged thyroid. The thyroid had heterogeneous echotexture and normal vascularity. There was a solid hypoechoic nodule on the right middle pole that measured 4 mm anteroposteriorly and was scored on the Thyroid Imaging, Reporting and Data System7 (TI-RADS) scale as TR4 (see below for more about TI-RADS scoring). There was also a nodule on the left middle pole that measured 0.9 × 1.4 × 0.9 cm, was noted as taller-than-wide (Figure 1), and was scored TR5 on the TI-RADS scale.
Thyroid ultrasonography images. (A) Transverse view of the right thyroid lobe showing a 4-mm nodule (blue arrow). (B) Transverse view of the left thyroid lobe showing a solid hypoechoic nodule that is taller-than-wide (see calipers and measurements).
Given the patient’s thyroid nodules and low TSH level, a radioactive iodine uptake and scan was obtained. After oral administration of 10.2 μCi iodine-131, 24-hour iodine uptake was 16% (7%–30%) with heterogenous distribution of the radiotracer throughout the thyroid gland. Thus, a diagnosis of subclinical hyperthyroidism due to toxic multinodular goiter was made.
The imaging was reviewed by a radiologist, who could not definitively categorize the 1.4-cm middle-pole nodule on the left as hot (tracer uptake is greater in the nodule than in the surrounding healthy thyroid tissue, indicating the nodule has an extremely low risk of malignancy8) or cold (less tracer uptake in the nodule than in the surrounding thyroid) but favored cold.
Because this nodule was scored as TR5 (highest risk category for cancer) and was larger than 1 cm, thyroid fine-needle aspiration (FNA) was indicated and performed. Initial cytology was nondiagnostic (Bethesda category 1) due to scant cellularity. An FNA was repeated 8 weeks later, and the cytology was atypia of undetermined significance (Bethesda category 3).
Although very rarely documented in the literature, performing an FNA can trigger worsening of thyrotoxicosis, including thyroid storm.9 Thus, the recommendation is to avoid FNA in overt hyperthyroidism; however, because our patient had subclinical hyperthyroidism, FNA was justified.
MANAGEMENT OF ATYPIA IN THYROID CYTOLOGY
2. What are the options for management of thyroid nodules with indeterminate cytology (Bethesda category 3 or 4)?
Diagnostic hemithyroidectomy
Repeat thyroid FNA
Radioactive iodine ablation
Molecular diagnostic testing
Thyroid nodules are very common—more than half the general population may have at least 1.10 Most are benign and nonfunctional; approximately 10% are cancerous.11 An ongoing challenge is determining which nodules are clinically significant thyroid cancer that, left untreated, would lead to morbidity or mortality.
TI-RADS scoring
Previous studies have shown papillary carcinomas that would not result in mortality or symptoms if left alone are overdiagnosed.12 Because of this overdiagnosis, in 2017 the American College of Radiology published the TI-RADS system to identify thyroid nodules that warrant FNA based on a reasonable likelihood of clinically significant cancer.7 Points are assigned based on a rubric covering 5 categories: nodule composition (ie, cystic vs solid), echogenicity, shape (ie, wider-than-tall vs taller-than-wide on transverse view), margins, and echogenic foci. Points are then totaled to determine a TI-RADS score or risk level. The risk level—TR1 to TR5—is then combined with nodule size to guide FNA decision-making. For example, a TR4 nodule has an aggregate risk of malignancy of 9.1% and a TR5 nodule has an aggregate risk of malignancy of 35%.13 Thus, patients with TR4 nodules undergo FNA when the nodules are 1.5 cm or larger and patients with TR5 nodules when the nodules are 1 cm or larger.
Bethesda system
The Bethesda System for Reporting Thyroid Cytology, which has been in widespread use for more than a decade, offers a standardized approach to describing and categorizing thyroid specimens from FNA.10
Bethesda category 1 (nondiagnostic) thyroid nodules are typically biopsied again14
Bethesda category 2 thyroid nodules have benign cytology
Bethesda category 3 or 4 (indeterminate cytology) is common and carries an intermediate risk of malignancy; management strategies include repeat FNA, use of additional molecular testing to stratify the nodule risk based on genetic profile, and diagnostic lobectomy or thyroidectomy
Bethesda 5 (suspicious for cancer) or 6 (consistent with cancer) nodules are typically managed with referral to a thyroid surgeon for hemithyroidectomy or total thyroidectomy.
CASE CONTINUED: MULTIDISCIPLINARY DISCUSSION
The patient was informed of the indeterminate cytology, and details of his case were discussed with the multidisciplinary endocrine tumor board, which included endocrinologists, otolaryngologists, and nuclear-medicine specialists. Because the patient strongly desired definitive therapy for all thyroid diseases, the consensus recommendation was thyroidectomy.
THYROIDECTOMY IN SUBCLINICAL HYPERTHYROIDISM
Thyroidectomy is not the typical first-line treatment of subclinical hyperthyroidism,6 nor is it the only option for thyroid nodules with indeterminate cytology,14 as reviewed above. However, radioactive iodine ablation was not considered given the patient’s personal history of acute lymphoblastic leukemia and because of the indeterminate thyroid cytology, as a potential thyroid cancer cannot be fully ablated without first performing a thyroidectomy. Medical treatment of subclinical hyperthyroidism with methimazole would not have addressed the indeterminate cytology, and the healthcare system where the patient was receiving care did not have access at that time to reliable molecular testing for indeterminate thyroid nodules.
Therefore, total or near-total thyroidectomy is a reasonable approach for multiple scenarios that apply to our patient: treatment of hyperthyroidism when there is coexisting structural disease and indeterminate cytology of the thyroid nodules. Referral to a high-volume thyroid surgeon is preferred. High-volume surgeons, described as performing more than 25 thyroid surgeries per year, have better clinical and cost patient outcomes than low-volume surgeons, who have 51% higher complication rates.15 Those complications can include postsurgical hypoparathyroidism and recurrent laryngeal nerve injury.
CASE CONTINUED: THYROIDECTOMY
Total thyroidectomy was performed 6 weeks later (approximately 5 months after initial presentation). Thyroid function was similar at the time of surgery: TSH 0.019 mIU/L and free T4 0.98 ng/dL.
The surgical pathology report indicated a small (< 1 mm) microscopic focus of nonencapsulated papillary thyroid carcinoma. The remaining background thyroid was reported as “no significant pathologic findings.” Two healthy parathyroid glands were removed and reimplanted in the neck. Small samples of each gland were sent to pathology, and the results were reported as “cellular parathyroid tissue” with “no evidence of malignancy.”
The patient was discharged home after the thyroidectomy on levothyroxine 200 μg daily based on his weight of 135 kg. Subsequently, he has had no clinical or biochemical evidence of hypoparathyroidism.
YET ANOTHER THYROID DIAGNOSIS
3. What is the next step in management given the finding on surgical pathology of papillary thyroid microcarcinoma?
Radioactive iodine ablation
Reoperation with lymph node dissection
Chemotherapy
No additional treatment
Even when thyroid surgery is explicitly performed for presumed benign thyroid disease, incidental papillary thyroid microcarcinoma is common and accounts for nearly 10% of cases.16 As both active surveillance and surgical excision are now considered reasonable strategies for papillary thyroid microcarcinoma (< 1 cm), the thyroidectomy that discovered the microcarcinoma is considered sufficient treatment.17 Such patients are not treated more aggressively (eg, radioactive iodine ablation, chemotherapy, or lymph node dissection) because papillary thyroid microcarcinomas generally have an excellent prognosis16 with low risk of structural recurrence.8
CASE CONTINUED: EYE SYMPTOMS DEVELOP
While awaiting thyroidectomy, the patient had developed periorbital edema on his right side. At that time, he had no blurry or double vision, excessive dryness, or tearing of the eyes. Thyroid eye disease (TED) was suspected. Results from repeat laboratory testing showed that his thyroid-stimulating immunoglobulin level was mildly elevated at 0.77 IU/L.
4. Which of the following are management options for TED?
Smoking cessation and avoidance of secondhand smoke
Lubricating eye drops
Avoidance of hypothyroidism
Radioactive iodine treatment of hyperthyroidism
THYROID EYE DISEASE
TED is associated with antibodies that bind the TSH receptor (eg, thyroid-stimulating immunoglobulin and thyrotropin receptor antibodies), which is present in orbital fibroblasts and forms a complex with the insulin-like growth factor 1 receptor.18 This process is not fully understood, but it seems to result in the activation of orbital fibroblasts and deposition of glycosaminoglycans in the extracellular matrix, causing swelling.19 Risk factors for TED include age 40 to 60 years, cigarette smoking, high thyroid-stimulating immunoglobulin or thyrotropin receptor antibody titers, and uncontrolled hyper- or hypothyroidism.
Radioactive iodine therapy may exacerbate TED, so it is typically avoided.19 Thionamides and thyroidectomy have not been shown to affect the course of TED. Mild-to-moderate TED is typically managed with local supportive measures, such as lubricating eye drops, and careful maintenance of a euthyroid state. Intravenous glucocorticoids have historically been used for moderate-to-severe active TED.
Teprotumumab, an insulin-like growth factor 1–receptor inhibitor, was approved by the US Food and Drug Administration in 2020 for moderate-to-severe active orbitopathy.20 Adverse effects of teprotumumab, which occur in 10% to 30% of patients, include sensorineural hearing loss, infusion reactions, and hyperglycemia.21 Hyperglycemia is more common in patients with preexisting diabetes mellitus and can persist after teprotumumab cessation.22
Orbital decompressive surgery is considered either emergently for vision-threatening manifestations of TED, such as optic neuropathy, or nonemergently to correct the sequelae of TED, such as severe proptosis with exposure keratopathy or strabismus.19
CASE CONTINUED: LONGITUDINAL MANAGEMENT
In the months after his thyroidectomy, the patient’s levothyroxine dose required frequent adjustment because he continued to have low TSH values on repeat testing. His TSH level did not normalize until 13 months after the thyroidectomy, when it was 0.384 mIU/L on levothyroxine 125 μg daily. However, his TSH levels rose as high as 14.516 mIU/L in the ensuing months, despite careful dose adjustment, patient adherence to proper administration, and frequent follow-up. His levothyroxine dose was titrated up by endocrinology, and he ultimately reached euthyroidism at 137 μg daily. However, this was accompanied by worsening TED symptoms, with an increase in pressure sensation, greater proptosis, and blurry vision in his left eye.
When his TSH level reached 14.516 mIU/L, the patient was evaluated by an oculoplastic surgeon, who did not find any optic nerve compromise and found normal visual acuity. Treatment included artificial tears, warm compresses, and vitamin D supplementation.
Teprotumumab continues to be considered; however, 2 years after the patient’s thyroidectomy, it has not yet been started because it is believed that the risks clearly outweigh the benefits for the patient.
CONCLUSION
This case shows how a patient can present with symptomatic subclinical hyperthyroidism but have several thyroid-related diagnoses, including thyroid nodules, incidental papillary thyroid microcarcinoma, and TED. Thyroid care is complex, especially when extrathyroidal manifestations of thyroid disease are present. Patients may require a team of specialists comprising endocrinologists, thyroid surgeons, nuclear medicine physicians, ophthalmologists, and oculoplastic surgeons.
TAKE-HOME POINTS
Subclinical hyperthyroidism (low TSH with normal T4 and T3) should be treated when symptomatic; when TSH is persistently less than 0.10 mIU/L in patients 65 or older; in patients with cardiac risk factors, heart disease, or osteoporosis; and in postmenopausal women who are not on estrogen or osteoporosis treatment.6
Thyroid nodules are best visualized by ultrasonography. If TSH is low, a radioactive iodine uptake and scan is also indicated to determine whether the nodule is hot, because those nodules are more likely benign.8
The TI-RADS scoring system, combined with nodule size, helps determine which nodules can be assessed by FNA.7
Papillary thyroid microcarcinomas (< 1 cm) are considered fully treated by hemithyroidectomy or thyroidectomy.17
TED is a common extrathyroidal manifestation of thyroid disease. It can threaten sight, so a high level of suspicion is required, with involvement of specialists as needed.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2025 The Cleveland Clinic Foundation. All Rights Reserved.