ABSTRACT
Severe COVID-19 illness is associated with intense inflammation, leading to high rates of thrombotic complications that increase morbidity and mortality. Markedly elevated levels of D-dimer with normal fibrinogen levels are the hallmark laboratory findings of severe COVID-19–associated coagulopathy. Prophylaxis against venous thromboembolism is paramount for all hospitalized patients with COVID-19, with more aggressive prophylaxis and screening recommended for critically ill patients with D-dimer levels above 3.0 μg/mL. Point-of-care ultrasonography is the imaging method of choice for patients at high risk, as it entails minimal risk of exposing providers to the virus.
We recommend measuring D-dimer, fibrinogen, prothrombin time, international normalized ratio, and activated partial thromboplastin time every 48 hours in hospitalized patients with COVID-19.
Prophylaxis against venous thromboembolism is recommended for all patients with COVID-19 on admission, using low-molecular-weight heparin, unfractionated heparin for those in renal failure, or fondaparinux for those with heparin-induced thrombocytopenia, even in the setting of thrombocytopenia as long as the platelet count is above 25 × 109/L.
Critically ill intensive care unit patients with D-dimer levels 3.0 μg/mL or higher should undergo screening with point-of-care ultrasonography and receive more intensive prophylaxis.
INTRODUCTION
COVID-19–associated coagulopathy (CAC) and disseminated intravascular coagulation are common in COVID-19 and are associated with severe illness and death.1–3 Critically ill patients without other risk factors for thrombosis can experience various thrombotic events, including microvascular thrombosis, venous and pulmonary thromboembolism, and acute arterial thrombosis.4
This article discusses clinical manifestations of CAC, associated laboratory and histologic findings, recent evidence elucidating pathophysiologic mechanisms, and the way we manage it at Cleveland Clinic.
A HIGHLY THROMBOTIC STATE
The clinical presentation of CAC is that of a highly thrombotic state. Shared anecdotal experience from a variety of sources indicates that catheter-associated thrombosis and clotting of vascular access catheters are frequently encountered. The need for catheter replacement and dialysis circuits that involve frequent interruption of continuous renal replacement therapy are other scenarios at high-risk for clotting and thrombosis development.
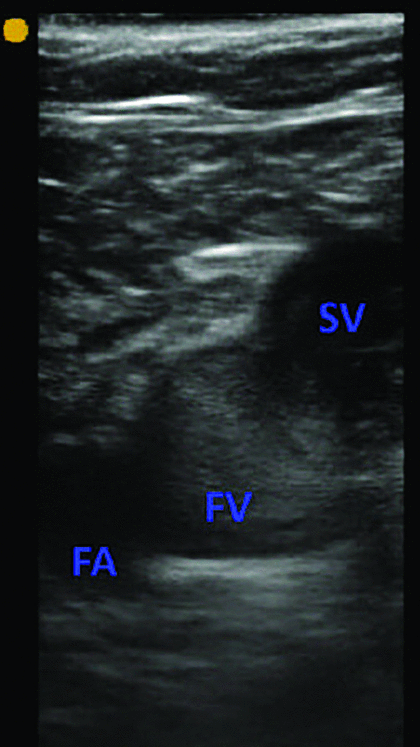
Two earlier studies support the clinical impression that COVID-19 is highly thrombotic. Cui et al5 reported a 25% incidence of deep vein thrombosis in patients with severe coronavirus pneumonia in Wuhan. In the Netherlands, Klok et al4 found a 31% combined incidence of deep vein thrombosis, pulmonary embolism, and arterial thrombosis in critically ill patients with coronavirus. Of these events, 81% were pulmonary thromboemboli. This is similar to our own clinical experience at Cleveland Clinic intensive care units (ICUs), where the prevalence of DVT is 25% to 30% on point of care ultrasound examination (POCUS). Although variable prevalence rates have been reported in subsequent studies, a recent meta-analysis confirmed a pooled prevalence rate of venous thromboembolism (VTE) of 31% in ICU patients despite pharmacologic VTE prophylaxis.6 Another frequent finding observed on POCUS is occurrence of spontaneous echo contrast (SEC); described as amorphous echogenicity in major veins. The presence of SEC has been associated with a higher subsequent risk of deep vein thrombosis (Figures 1–3).7,8
A short-axis view of the femoral vein (FV) and the femoral artery (FA) at the site of the saphenous vein (SV) inflow. Amorphous echogenicity in the femoral vein, greater than that of the adjacent femoral artery, is suggestive of slow venous flow. The vein was fully compressible, ruling out deep vein thrombosis at the site.
Figure 2 (video). Another short-axis view of the femoral vein (center) and the femoral artery (bottom right) at the site of the saphenous vein inflow (top right). Swirling pattern of high echogenicity suggests low-flow state.
Figure 3 (video). Long-axis view of the femoral vein, with spontaneous echogenicity and slow flow.
LABORATORY FINDINGS: ELEVATED D-DIMER
The characteristic laboratory findings of CAC are significantly elevated levels of D-dimer, fibrin degradation products and fibrinogen, indicating a highly thrombotic state with high fibrin turnover.9 However, other markers of disseminated intravascular coagulation remain relatively unchanged.10 The prothrombin time and activated partial thromboplastin time are only mildly prolonged, if at all, and platelet counts are usually normal or marginally reduced (100–150 × 109/L).9,11,12 Abnormalities in coagulation parameters have been shown to correlate directly with inflammatory cytokines, severity of illness and adverse outcomes.13,14
Elevated D-dimer
Elevated D-dimer levels on presentation with COVID-19 are a hallmark of more severe disease. Levels of 0.5 μg/mL or higher were found in 59.6% of patients with severe disease vs 43.2% of those with mild disease.3 High levels also correlated with the need for intensive care15 and with death.
In a multivariable regression analysis of 191 patients, Zhou et al1 reported that the risk of death was more than 18 times higher (odds ratio 18.42, 95% confidence interval 2.64–128.55) for patients admitted with a D-dimer level greater than 1 μg/mL vs less than 0.5 μg/mL. Cui et al5 reported that D-dimer levels also correlated with risk of venous thromboembolism: a level of 3.0 μg/mL had a sensitivity of 70.0%, specificity of 96.7% with a positive predictive value of 87.5%. Maatman et al16 reported that standard prophylaxis against venous thromboembolism failed in 29 of 109 patients in the ICU, and of those in whom it failed, all had D-dimer levels greater than 3.0 μg/mL.
Fibrinogen
Fibrinogen is characteristically elevated in patients with severe COVID-19. Fibrinogen levels correlate with levels of IL-617 and severity of disease.18 Levels appear to be elevated throughout the course of the disease, but an acute drop late in the course may signal the onset of acute consumptive coagulopathy and be an indicator of particularly poor prognosis.9
Prothrombin time, activated partial thromboplastin time
Klok et al4 did not report D-dimer levels, but found coagulopathy (ie, prolongation of prothrombin time of > 3 seconds or of activated thromboplastin time > 5 seconds) to be an independent risk factor for thrombosis. Prolongation of PT has also been shown to correlate with more severe disease, greater risk for organ failure (such as ARDS and renal failure) and increased mortality.13
Antiphospholipid antibodies
Zhang et al19 reported that 3 patients with CAC and lower-extremity ischemia had antiphospholipid antibodies (anticardiolipin immunoglobulin A [IgA], anti-beta-2 glycoprotein 1 IgA and IgG) but not lupus anticoagulant. Helms et al,20 in a multicenter study of 150 patients with COVID-19 in ICUs in France, found a remarkably high rate of positivity for lupus anticoagulant: 50 of 57 patients (87.7%) among those tested for further evaluation for an elevated activated partial thromboplastin time. Preliminary studies suggest that these antibodies may induce neutrophil extracellular trap release from neutrophils.21
Disseminated intravascular coagulation score
Tang et al9 found that progression of coagulopathy to overt disseminated intravascular coagulation (defined by the International Society on Thrombosis and Haemostasis as a disseminated intravascular coagulation score ≥ 5 points; the score is based on platelet count, D-dimer level, fibrinogen level, and prolongation of the prothrombin time) predicted a poor prognosis, occurring in 71.4% of all nonsurvivors vs 0.6% of survivors.
Progressive consumptive coagulopathy
Declining levels of antithrombin III, a rise in prothrombin time and activated partial thrombolastin time, and dramatic further increase of D-dimer (> 15.0 μg/mL) appear to indicate severe and progressive disease, developing late in the disease course (day 10 to 14) of nonsurvivors. Fibrinogen levels, which are elevated in the initial phase, drop late in the course of disease in nonsurvivors and may signal impending death.9
Low platelet count
Lippi et al,11 in a meta-analysis of 9 studies with 1,779 patients with COVID-19, examined thrombocytopenia as a marker of disease severity. Thrombocytopenia at presentation was associated with an increased risk of severe disease and death, with a weighted mean difference of 31 × 109/L in the platelet count between those with severe and nonsevere disease. The authors noted great heterogeneity among studies, with reported rates of thrombocytopenia in severe disease ranging from 4% to 57.7%.
Viscoelastic testing
Viscoelastic test such as Rotational Thromboelastometry (ROTEM) and Thromboelastography (TEG) are “global” tests of clot formation, clot strength and clot lysis. Several studies have shown a “hypercoagulable profile” on viscoelasctic testing, including faster clot formation, increased clot strength and a decrease in clot lysis.17,22–25 A hypercoagulable profile on viscoelastic testing may be associated with an increased risk of thrombosis despite adequate prophylaxis and organ failure.
SEVERE LUNG DAMAGE FROM INFLAMMATION, THROMBOSIS
Histopathologic studies reveal diffuse alveolar damage with profound inflammation, thrombosis, and thrombotic microangiopathy of small vessels and capillaries of the lung. Also noted have been megakaryocytes within pulmonary capillaries with nuclear hyperchromasia and atypia, as well as neutrophils partially degenerated and entrapped in fibers (suggesting neutrophil extracellular traps).26 An autopsy series of 11 patients showed thrombosis of small and midsized pulmonary arteries in all patients.27
Endothelial cell injury and diffuse microvascular thrombosis suggestive of thrombotic microangiopathy have also been reported in extrapulmonary organs, which may explain the acute onset of multiorgan failure without an otherwise obvious etiology.28
PATHOPHYSIOLOGY: INFLAMMATION PROMOTES THROMBOSIS
CAC is likely multifactorial, and patients with COVID-19 share many of the classic risk factors for venous thromboembolism seen in adult respiratory distress syndrome from other causes, such as immobility, large vascular-access catheters, and systemic inflammation.
The hallmark of COVID-19 is profound inflammation, described as “cytokine storm,” characterized by high levels of interleukin 1 (IL-1), IL-6, tumor necrosis factor, and other inflammatory cytokines.15 Inflammation promotes thrombosis through various mechanisms, including activation of endothelial cells, platelets, monocytes, and the tissue factor-factor VIIa pathway, and by altering fibrinolysis and natural anticoagulant pathways (eg, through changes in levels of thrombomodulin, proteins C and S, and tissue-factor-pathway inhibitor).29,30
Multiple studies have demonstrated a direct correlation between cytokine levels and laboratory marketers of coagulopathy coagulopathy.13,14 Intense inflammation with thrombosis of pulmonary vessels is also seen in adult respiratory distress syndrome of other etiologies.31 It remains to be seen if these findings represent a distinct phenotype unique to COVID-19 or are a general indicator of the severity of inflammation with COVID-19.
Serum proteomic profiling of patients with severe acute respiratory syndrome (SARS) identified an N-terminal fragment of complement C3C-alpha (a central component of the complement pathway) as a sensitive biomarker of early SARS.32 Murine mod els of SARS and Middle East respiratory syndrome (MERS) have shown that complement activation is a major contributor to lung injury and other organ failure. Complement inhibition in these models reduced organ damage and inflammation.33,34 Complement inhibition has been suggested as a treatment for COVID-19, but clinical data are not yet available.35 The SARS-CoV-2 spike protein activates complement in vitro via the alternative pathway.36
One mechanism of microvascular thrombosis that may be specific to COVID-19 is the virus’s affinity for angiotensin-converting enzyme 2, which is expressed on alveolar epithelial type II cells and various extrapulmonary tissues, including endothelial cells. SARS-CoV-2 has been shown to directly invade the endothelial cell.37 Endothelial cell activation may be a unique mechanism of COVID-19-mediated microvascular injury, thrombosis, and subsequent multisystem organ failure and is believed to be a major contributor to coagulopathy, morbidity and mortality.37–40
The rate of 87.7% positivity for lupus anticoagulant in patients with COVID-19 reported by Helms et al20 is striking and needs to be verified, but it supports the idea that endothelial injury is a key mechanism of multiorgan failure and coagulopathy in this disease. The “two-hit” model of thrombosis associated with antiphospholipid syndrome proposes that after a first-hit injury to the endothelium, antiphospholipid antibodies potentiate thrombus formation as a second hit.41 Activation of the contact system due to increased vascular permeability and thrombotic microangiopathy warrant further exploration.42
OUR MANAGEMENT APPROACH
Currently, CAC is managed largely on the basis of anecdotal experience and cohort studies; controlled studies are currently underway but high quality prospective data is still lacking and is urgently needed to better guide care.
This lack of data is reflected in the conflicting recommendations from the various guidelines for the management of thrombotic risk associated with severe COVID-19.
The current CHEST guidelines recommend standard VTE prophylaxis.43 In contrast, an expert panel of the American College of Cardiology failed to reach consensus on a recommendation for standard versus intensified prophylaxis or empiric therapeutic dose anticoagulation,44 while the authors of the interim guidance from the anticoagulation forum recommend high intensity prophylactic dosing for all critically ill patients.45 Our approach represents an attempt to balance the potential risks and benefits of intensified prophylaxis by selecting patients at greatest risk for thrombosis for intensified prophylaxis. As we await the results of prospective randomized controlled trials, we are assured by recent reports of low overall bleeding risk even with empiric therapeutic anticoagulation.46
Monitor D-dimer, fibrinogen, prothrombin time, activated partial thromboplastin time
In view of the characteristic laboratory findings of CAC described above, we monitor D-dimer, fibrinogen, prothrombin time-international normalized ratio, and activated partial thromboplastin time every 48 hours. We define a D-dimer level of at least 6 times the upper limit of normal (3.0 μg/mL fibrinogen equivalent units [FEU]) as high risk.5,9
Because antiphospholipid antibodies, including lupus anticoagulant, have been reported in COVID-19, we recommend testing for these if the activated partial thromboplastin time is spontaneously elevated, and we prefer the use of anti-Xa assays to monitor anticoagulation in patients on therapeutic anticoagulation. Anti-Xa assays however, may be affected by high levels of bilirubin (> 6.6 mg/dL) or triglycerides (> 360 mg/dL),47 which are often elevated in patients with COVID-19 and cytokine storm. Adding to the risk of hypertriglyceridemia is the use of propofol, a lipid emulsion, for sedation in mechanically ventilated patients. Triglyceride levels should therefore be monitored routinely and considered as a possible source of error in patients on anticoagulation who are difficult to maintain within the therapeutic target range.
A hypercoagulable pattern on viscoelastic testing (thromboelastography or rotational thromboelastometry), with faster time to clot formation, rapid clot propagation, and increased clot strength, has been described in several publications.16,17 However, no evidence exists on how to best use this information to guide therapy. In line with current recommendations from various societies and expert panels, we do not routinely use viscoelastic testing to assess hypercoagulability.43,48
Imaging: Use POCUS
To limit caregiver exposure, we minimize formal bedside vascular studies and sending the patient out of the ICU for computed tomographic angiography. We rely heavily on POCUS to assess for evidence of venous thromboembolism.49 This is in line with recent recommendations by CHEST and the National Institutes of Health guidance,43,50 which cites a lack of evidence supporting routine screening examinations but highlights the value of POCUS in the hands of experienced clinicians. POCUS should be bundled with other care (for example, ultrasonography-guided vascular access) to minimize the use of personal protective equipment and caregiver exposure to COVID-19.
We perform POCUS evaluation in patients with new or acutely worsening hypoxia as well as those who fail to improve or have laboratory indicators of poor prognosis or increase risk of thrombosis. We have defined an elevated D-Dimer level > 6x the upper limit of normal (D-dimer > 3.0 μg/mL FEU) as high risk.
Patients at high risk are assessed for deep vein thrombosis using a 3-point compression POCUS examination of both lower extremities. A POCUS deep vein thrombosis examination and echocardiography are also recommended for any patient with sudden cardiopulmonary decline that cannot be explained by an alternative etiology.
A positive POCUS examination for deep vein thrombosis is highly specific and does not need to be confirmed by formal vascular ultrasonography.51 On the other hand, given the high incidence of pulmonary embolism described, confirmatory studies (ie, formal vascular ultrasonography or computed tomographic angiography) are warranted if the patient has contraindications to empiric anticoagulation and the clinical suspicion of venous thromboembolism is high despite negative POCUS, or if POCUS is not available.
Prophylactic heparin for most
Specific data on the management of CAC are extremely limited, but given the high risk of thrombotic complications, heparin or low-molecular-weight heparin prophylaxis is currently recommended for all acutely hospitalized patients who do not have specific contraindications.
In addition to its antithrombotic effect, heparin may have anti-inflammatory, anticomplement,52 and direct antiviral effects that may be beneficial in COVID-19. Heparin inhibits neutrophil activation, binds inflammatory cytokines, and reduces endothelial activation.53 Experimental models have also shown that heparin directly binds to SARS-CoV spike protein, the viral anchor site, thereby blocking viral entry into the cell.54 While promising, these effects have yet to be demonstrated clinically.
Tang et al55 reported on 449 patients with severe COVID-19 in whom the overall mortality rate was no different (29.7% vs 30.3%, P = .910) between those who received heparin (94 patients on low-molecular-weight heparin, 5 patients on unfractionated heparin; prophylactic doses) and those who did not. But among patients with a D-dimer level of more than 6 times the upper limit of normal (> 3.0 μg/mL), heparin recipients had a significantly lower mortality rate than nonrecipients (32.8% vs 52.4%, P = .017). The authors concluded that heparin lowers mortality rates in patients with severe COVID-19 and cited a Chinese consensus statement recommending anticoagulation in severe COVID-19. We emphasize that this study retrospectively compared heparin prophylaxis with no prophylaxis.
Full anticoagulation for some?
Some evidence indicates a higher rate of “heparin failure” in patients with COVID-19.56 Elevated D-dimer levels may predict higher risk of venous thromboembolism despite standard prophylaxis. In a study of 240 critically ill patients with COVID-19, Maatman et al16 reported a 28% rate of venous thromboembolism in patients receiving standard prophylaxis. Elevated D-dimer (> 2.6 μg/mL) predicted venous thromboembolism with a sensitivity of 89.7%. The authors concluded that standard prophylactic anticoagulant doses may be insufficient to prevent venous thromboembolism in high-risk patients.
Paranjpe et al,57 in an observational report of 2,773 patients with COVID-19 admitted to a single institution in New York, found that those treated with full anticoagulation (786 patients, 28%) had a similar mortality rate (22.5%) vs those treated with prophylaxis only (22.8%). But among mechanically ventilated patients, in-hospital mortality was 29.1% for those treated with anticoagulation vs 62.7% for patients who did not receive anticoagulation. Despite this dramatic reduction of mortality, the authors advise caution in applying these findings, given the serious limitations of the report, ie, its observational nature and lack of information on illness severity and indications for anticoagulation.
Taken together, this limited evidence confirms that prophylaxis against venous thromboembolism in critically ill COVID-19 patients is associated with improved outcomes, and there may be a role for intermediate dose or even full anticoagulation.
Given the limitations of the studies thus far, it remains unclear if higher prophylactic doses, intermediate doses or full anticoagulation, offer a benefit beyond standard prophylactic dosing, and which patients may benefit without suffering more bleeding complications.
Thrombolysis has also been suggested for patients whose condition deteriorate despite anticoagulation. Several case reports and case series describe improvement in oxygenation after treatment with thrombolysis in patients with persistent severe hypoxia, markedly elevated D-dimer or proven thromboembolism.58 Most reports do not describe significant bleeding complications, but mortality among these critically ill patients remains high.59 Clinical trials to evaluate the role of thrombolytic therapy are currently ongoing.
Recommendations
Given this lack of evidence, the National Institutes of Health, American Society of Hematology, and International Society on Thrombosis and Haemostasis currently do not recommend treatment beyond standard prophylaxis except for an established indication. Prophylaxis against deep vein thrombosis is strongly recommended in all patients on admission, using low-molecular-weight heparin (or unfractionated heparin in those with renal failure, or fondaparinux in those with heparin-induced thrombocytopenia). Current guidance also stresses that prophylaxis should be continued even in the setting of thrombocytopenia so long as the platelet count is higher than 25 × 109/L.48,60 Intensified pharmacologic prophylaxis for ICU patients at greatest risk is also recommended by some.45
Our current approach is to provide standard dose pharmacological VTE prophylaxis to all patients with COVID-19 that require inpatient admission. In critically ill patients requiring ICU admission, we adjust therapy based on POCUS screening for venous thromboembolism and intensified prophylaxis in high-risk patients (Table 1, Figure 4). We divide patients into 3 categories:
Cleveland Clinic approach to anticoagulation prophylaxis and management in COVID-19
Algorithm for preventing and treating COVID-19-associated coagulopathy.
FEU = fibrinogen equivalent units
Category 1: D-dimer less than 3.0 μg/mL FEU and no evidence of venous thromboembolism. Patients receive standard prophylaxis and are monitored using serial D-dimer testing.
Category 2: D-dimer 3.0 μg/mL FEU or higher, POCUS-negative. Patients receive intensified deep vein thrombosis prophylaxis.
Category 3: Confirmed thrombosis. Patients receive full anticoagulation.
If the clinical suspicion of venous thromboembolism is high and the patient has no contraindication to anticoagulation, full anticoagulation should be initiated empirically if POCUS or confirmatory tests are not immediately available.
Continuous renal replacement therapy
Given the high rate of clotting in dialysis circuits, all patients on continuous renal replacement therapy receive unfractionated heparin at a rate of 500 U per hour. If ongoing clotting is observed, we increase systemic heparin to bring the activated partial thromboplastin time into the target range according to an acute coronary syndrome nomogram. The target activated partial thromboplastin time is 49 to 67 seconds, and the goal anti-factor Xa level is 0.2 to 0.5 IU/mL, but these may be adjusted if clotting continues despite systemic heparin.
Duration of anticoagulation
Anticoagulation should be continued for 6 weeks for catheter-associated thrombosis and for at least 3 months for venous thromboembolism. Convalescent patients with persistently elevated D-dimer (greater than twice the upper limit of normal) may benefit from extended prophylaxis or treatment.61,62
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Footnotes
The statements and opinions expressed in COVID-19 Curbside Consults are based on experience and the available literature as of the date posted. While we try to regularly update this content, any offered recommendations cannot be substituted for the clinical judgment of clinicians caring for individual patients.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.