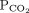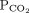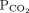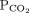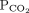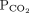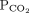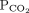Article Figures & Data
Tables
Substance Value Reference range Blood chemistry Sodium 132 mmol/L 136–144 Potassium 4.8 mmol/L 3.7–5.1 Bicarbonate 16.0 mmol/L 22–30a Chloride 95 mmol/L 97–105 Blood urea nitrogen 23 mg/dL 7–21 Creatinine 1.3 mg/dL 0.58–0.96 Glucose 97 mg/dL 74–99 Lactate 1.1 mmol/L 0.5–2.2 Albumin 4.5 g/dL 3.9–4.9 Serum osmolality 318 mOsm/kg 275–295 Arterial blood gases pH 7.25 7.35–7.45 
28 mm Hg 34–46b Bicarbonate 16 mmol/L 22–26 1 Determine the arterial pH status pH < 7.40 is acidemic, pH > 7.44 is alkalemic
But a normal pH does not rule out an acid-base disorder2 If the arterial pH is abnormal, determine whether the primary process is respiratory, metabolic, or both pH 
Bicarbonate Respiratory acidosis Low High — Metabolic acidosis Low – Low Mixed respiratory and metabolic acidosis Low High Low Respiratory alkalosis High Low — Metabolic alkalosis High – High Mixed respiratory and metabolic alkalosis High Low High 3 Calculate the anion gap Anion gap = sodium – (chloride + bicarbonate) If serum albumin is low, add 2.5 mmol/L to the anion gap for every 1 g the serum albumin is below normal An anion gap > 10 mmol/L is elevated 4 Check the degree of compensation (respiratory or metabolic)  and bicarbonate should move in the same direction
and bicarbonate should move in the same directionNominal normal levels: bicarbonate 25 mmol/L and  mm Hg
mm HgIn respiratory acidosis, for every 10-mm Hg increase in  , bicarbonate should increase by 1 mmol/L in the first 48 hours and 4 mmol/L afterward
, bicarbonate should increase by 1 mmol/L in the first 48 hours and 4 mmol/L afterwardIn metabolic acidosis, for every 1-mmol/L decrease in bicarbonate,  should decrease by 1.3 mm Hg
should decrease by 1.3 mm HgIn respiratory alkalosis, for every 10-mm Hg decrease in  bicarbonate should decrease by 2 mmol/L in the first 48 hours and 5 mmol/L afterward
bicarbonate should decrease by 2 mmol/L in the first 48 hours and 5 mmol/L afterwardIn metabolic alkalosis, for every 1-mmol/L increase in bicarbonate,  may increase by 0.6 mm Hg
may increase by 0.6 mm Hg5 If the patient has metabolic acidosis with an elevated anion gap, check whether the bicarbonate level has decreased as much as the anion gap has increased In metabolic acidosis, the anion gap should increase by the same amount that bicarbonate decreases; a difference in these two changes is called a delta gap  = partial pressure of carbon dioxide
= partial pressure of carbon dioxideBased on information in reference 1
Then Now Methanol Methanol Uremia Uremia Diabetic ketoacidosisa Diabetic ketoacidosis Paraldehyde Pyroglutamate and propylene glycolc Isoniazid Isoniazid and ingestions Lactic acidemia (L and D)b Lactic acidemiad Ethylene glycol (di)Ethylene glycol Salicylates Salicylates ↵a Ketoacidosis can be a complication of alcoholism (betahydroxybutyric acid).
↵b d-Lactate is a consequence of short-bowel syndrome. If more common causes of metabolic acidosis are not present and the patient has a history suggesting short-bowel syndrome, d-lactate can be considered.
↵c If the patient is older, female, and has a history of acetaminophen ingestion—and if more common causes of metabolic acidosis are not present—consider pyroglutamic acid metabolic acidosis as an etiology.
↵d d-Lactate can also be a consequence of propylene glycol metabolic acidosis.
The osmol gap is the difference between the calculated serum osmolality and measured osmolality Calculated serum osmolality =
(Sodium x 2) + (Glucose/18) + (Blood urea nitrogen/2.8)An elevated osmol gap indicates osmotically active solutes in plasma that are not typically present under normal conditions, such as ethylene glycol, diethylene glycol, methanol, and their many metabolic products