ABSTRACT
Radiofrequency ablation has become a safe and effective treatment for atrial fibrillation. We believe that referral to an electrophysiologist for consideration of ablation may allow for better rhythm control and outcomes by altering the natural history of atrial fibrillation progression.
Atrial fibrillation is increasing in prevalence with the aging of the US population and is associated with worsening quality of life and increased risk of stroke, heart failure, and death.
Atrial fibrillation results in adverse atrial remodeling and fibrosis, eventually leading to persistence of the arrhythmia and making rhythm control difficult.
Catheter ablation has evolved to be a safe procedure with technologic advancements, especially in experienced tertiary care centers.
The primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, it could also decrease the risk of stroke, heart failure, and death, but these outcomes have not been systematically evaluated in a large randomized controlled trial.
A 64-year-old man with hypertension but without known structural heart disease presents for a second opinion on management of his atrial fibrillation. The condition was first diagnosed at age 38, when he experienced palpitations and shortness of breath on exertion; at times he also experienced decreased endurance and fatigue without overt palpitations. At first, these episodes occurred about twice a year, and the patient was managed with a beta-blocker for rate control and an oral anticoagulant.
Over the past 10 years, the episodes have become more frequent and longer-lasting and have required frequent cardioversions. He was given flecainide for rhythm control but continued to have frequent episodes, and so about 1 year ago he was switched to amiodarone, which controlled his rhythm better. However, after reading about side effects of amiodarone, he decided to seek a second opinion.
He was evaluated by our team and eventually underwent radiofrequency ablation. During the procedure, he was noted to have diffuse scarring and fibrosis of his left atrium, and afterward he continued to require antiarrhythmic drugs to maintain sinus rhythm.
Should he have been referred sooner? What factors should primary care physicians consider when referring a patient with atrial fibrillation for ablation?
THE EPIDEMIC OF ATRIAL FIBRILLATION
Atrial fibrillation is a large and growing public health problem. In 2010, it was estimated to affect 2.7 to 6.1 million people in the United States, and with the rapid aging of our population, its prevalence is expected to rise to between 5.6 and 12 million by 2050.1–3 It is associated with significant morbidity, poor quality of life, and increased risk of death, heart failure, stroke, and cognitive impairment.
The number of new cases per year has increased over the years despite research and preventive measures, which may reflect aging of the population and increased survival rates in patients with cardiovascular or comorbid conditions.1,4
Thus, atrial fibrillation is one of the most common cardiovascular conditions encountered by primary care physicians and cardiologists, putting them at the forefront of its management. Proper treatment in its early stages and referral to a specialist for advanced management may alter its natural history and improve clinical outcomes.
HOW DOES ATRIAL FIBRILLATION ARISE AND PERSIST?
Much is still unknown about the pathogenesis of atrial fibrillation, but considerable progress has been made in the past few decades, opening the door for clinical ablative strategies.
Multiple wavelet hypothesis
Until the late 1980s, the most widely accepted conceptual mechanism of atrial fibrillation was the multiple wavelet hypothesis developed by Moe et al.5 According to this hypothesis, atrial fibrillation begins with multiple independent wavelets occurring simultaneously and spreading randomly throughout both atria, and it persists if there are a minimum number of coexisting wavelets, increased atrial mass, and heterogeneous conduction delays across the atrial tissue.
The surgical maze procedure, in which a series of incisions arranged in a maze-like pattern is created in the left atrium, was predicated on this model. The theory was that these surgical lesions would compartmentalize the atria into discrete electrical segments and thereby reduce the number of circulating random wavelets.6,7
However, experimental and clinical studies suggest that although randomly propagating wavelets can contribute to maintaining atrial fibrillation, focal triggers are noted in most cases.
Focal triggers
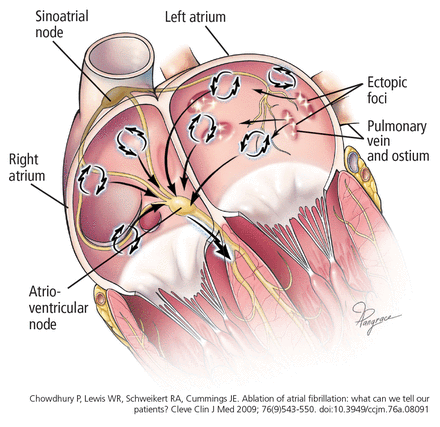
In 1997, Jaïs et al8 observed that atrial fibrillation is often triggered by a rapidly firing ectopic focus and that ablation of that focus can eliminate it. These ectopic foci are often found at or near the ostia of the pulmonary veins or near the superior vena cava.8,9 It is now well established that ectopic foci in the pulmonary veins are crucial triggers that initiate atrial fibrillation.
Trigger-and-substrate theory
Currently, the most widely accepted theory is that atrial fibrillation requires both a trigger and a susceptible substrate (Figure 1). Triggers consist of rapidly firing foci, most commonly located in the pulmonary veins but also in the superior vena cava, posterior wall of the left atrium, the vein and ligament of Marshall, the coronary sinus, and the left atrial appendage.
Atrial fibrillation is currently thought to arise from focal triggers, many of which are located in the pulmonary veins, and to be maintained by an abnormal substrate, ie, scarring and fibrosis of the left atrium.
The substrate for maintaining atrial fibrillation consists of an abnormal left atrium with heterogeneous fibrosis (scarring) and conduction delays. Any heart disease that increases left atrial pressure could lead to atrial dilation and remodeling, which could be substrates for atrial fibrillation. Extensive atrial remodeling and scarring are associated with progression and persistence of atrial fibrillation and make rhythm control more challenging.
Atrial fibrillation begets atrial fibrillation
As shown in the case above, over time, paroxysmal atrial fibrillation often progresses to persistent and long-standing atrial fibrillation if not aggressively managed initially.
In 1972, Davies and Pomerance10 performed 100 autopsies and found that the people who had had atrial fibrillation for longer than 1 month had lost muscle mass in the sinus node and internodal tract, and their atria were dilated. The study introduced the concept that atrial fibrillation itself causes pathologic changes in the atrium.
Wijffels et al,11 in an experiment in goats, showed that atrial fibrillation produced by rapid bursts of atrial pacing was initially paroxysmal. However, as they continued to induce atrial fibrillation over and over again, it lasted progressively longer until it would persist for more than 24 hours. Thus, in a relatively short time, the atria went from supporting paroxysmal fibrillation to supporting persistent fibrillation.
Atrial fibrillation leads to electrophysiologic and anatomic remodeling in the atrium, which leads to a shorter action potential duration and a shorter refractory period. This in turn makes it easier for atrial fibrillation to persist.12
Because atrial fibrillation tends to progress, intervening early may improve its outcomes. Early ablation has been shown to improve the chances of staying in sinus rhythm in both paroxysmal and persistent atrial fibrillation.13–15
CATHETER ABLATION OF ATRIAL FIBRILLATION
The goal of ablation is to prevent atrial fibrillation by eliminating the trigger that initiates it, altering the arrhythmogenic substrate, or both.
Pulmonary vein isolation
The most common ablation strategy is to electrically isolate the pulmonary veins by creating circumferential lesions around their antra. This creates a nonconducting rim of scar tissue, electrically disconnecting the pulmonary veins from the atrium.
Ablation outside of the pulmonary veins
Because recurrence rates are high in patients with persistent atrial fibrillation who undergo pulmonary vein ablation alone, the search continues for adjunctive strategies to improve outcomes. Although these strategies have a sound rationale based on experimental data and anecdotal evidence in humans, they have not yet been convincingly shown to be helpful in large clinical studies. Nonetheless, it is possible that more extensive substrate ablation—atrial “debulking”—could improve outcomes by reducing the amount of tissue that can fibrillate.
Linear ablation
Creating lines of ablation (as in the maze procedure) isolates different segments of the left atrium. Often, these lines are created along the roof of the left atrium between the right and left upper pulmonary veins and from the mitral valve to the left inferior pulmonary vein. The benefit of linear ablation has not been proven, and gaps in such lines may introduce atrial flutter.
Triggers not in the pulmonary veins
Common sites of nonpulmonary vein triggers include the posterior wall of the left atrium, the superior vena cava, the coronary sinus, and along the ligament of Marshall. Provocative maneuvers such as isoproterenol infusion can help find those triggers, which can then be ablated. A limitation is that there is no protocol proven to reproducibly elicit triggers.
Complex fractionated atrial electrograms are areas in the atrium with highly fractionated, low voltage potentials. They may be critical sites of substrate for atrial fibrillation, and many electrophysiologists target them in patients with persistent atrial fibrillation. But despite initial enthusiasm, doing so has not resulted in better outcomes in persistent atrial fibrillation.
Rotors
Animal studies have shown that atrial fibrillation can be triggered or maintained by localized sources of organized reentrant circuits (rotors) or focal impulses. Recent studies have shown that these electrical rotors and focal sources could potentially be mapped and ablated in humans. But positive results in initial reports have not been reproduced, and this remains an area of controversy.
Our practice
We isolate the pulmonary veins with antral ablations, ablate the posterior wall, and extend the ablation toward the septum and inferior to the right pulmonary veins, with good long-term outcomes.14 The rationale behind ablating the posterior wall is that it shares embryologic origins with the pulmonary veins and may be a common source of triggers in atrial fibrillation.
We do not routinely create empiric ablation lines in the left or right atrium unless the patient has atrial flutter. Empiric ablation lines have not been convincingly shown to provide additional benefit compared with our extensive ablation approach, which involves the posterior wall. Empiric ablation of the appendage or coronary sinus is typically reserved for repeat ablation in patients with recurrent persistent atrial fibrillation.
RATIONALE FOR TREATING ATRIAL FIBRILLATION WITH ABLATION
To control symptoms
At this time, the primary aim of atrial fibrillation ablation is to reduce symptoms and improve quality of life. In theory, ablation could also decrease the risk of stroke, heart failure, and death. However, these outcomes have not been systematically evaluated in any large randomized controlled trial.
To control rhythm and improve survival
Randomized controlled trials of rhythm vs rate control of atrial fibrillation16–18 have failed to demonstrate that restoring sinus rhythm is associated with better survival. All of these trials used antiarrhythmic drugs for rhythm control. However, nonrandomized studies19,20 showed that maintaining sinus rhythm is associated with a significant reduction in mortality rates, whereas the use of antiarrhythmic drugs increased mortality risk.
This suggests that the beneficial effect of restoring sinus rhythm may be offset by adverse effects of antiarrhythmic drugs, and if rhythm control could be achieved by a method other than antiarrhythmic drug therapy, it may be superior to rate control. On the other hand, these data may be affected by residual confounding. This topic deserves further research, but maintaining sinus rhythm is typically preferred whenever possible.
Discontinuing anticoagulation is not a goal at this time Retrospective studies have reported a low risk of stroke in patients who discontinue anti-coagulation several months after undergoing atrial fibrillation ablation.21–23 However, atrial fibrillation can recur, and risk of stroke increases with age.
Therefore, guidelines24 still recommend continuing anticoagulation after ablation. Generally, we do not offer ablation with a goal of discontinuing anticoagulation. That said, stopping anticoagulation may be considered after long-term suppression of paroxysmal atrial fibrillation on a case-by-case basis in patients deemed to be at low risk. Left atrial appendage closure devices may eventually allow concomitant atrial fibrillation ablation and closure of the appendage, so that anticoagulation could then be stopped. This remains a topic of investigation.
Who should be considered for ablation?
There are no absolute age or comorbidity contraindications to ablation. Everyone who has atrial fibrillation deserves, in our opinion, a referral to the electrophysiology clinic.
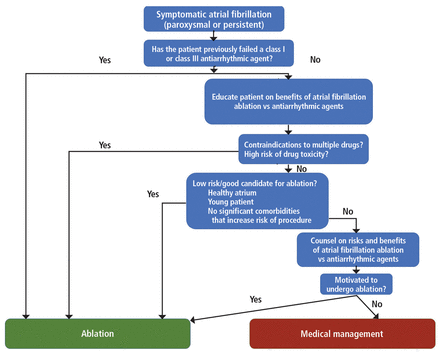
The decision to pursue ablation as opposed to trying drugs is nuanced, and needs a proper discussion with an electrophysiologist. The discussion of risks, benefits, and alternatives and the shared decision-making process before a patient undergoes ablation is the most time-consuming process in our clinic. Figure 2 shows our approach to deciding between ablation and medical management of atrial fibrillation.
Ablation vs medical management of atrial fibrillation. Most electrophysiologists in our institution use this general approach to decision-making.
PROCEDURAL CONSIDERATIONS
Atrial fibrillation ablation is most often performed by electrophysiologists using a minimally invasive endovascular approach. The patient can be under either moderate sedation or general anesthesia; we prefer general anesthesia for patient comfort, safety, and efficacy.
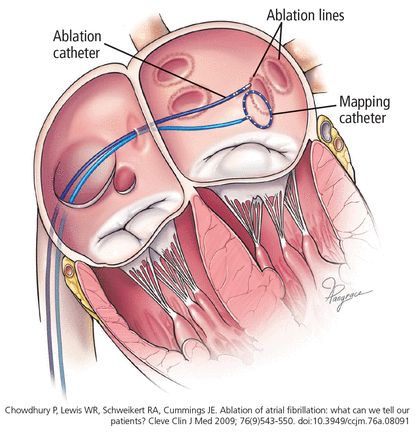
The catheter is inserted into the femoral vein and advanced into the right atrium. The interatrial septum is punctured under fluoroscopic and intracardiac echocardiographic guidance. Once the catheter is inside the left atrium, the antra of the pulmonary veins are located, and antral ablation is performed to electrically isolate the pulmonary veins from the atrial myocardium (Figure 3, Figure 4).
Radiofrequency ablation of atrial fibrillation.
Fluoroscopic view of radiofrequency ablation. The PentaRay catheter is used to acquire data for 3-dimensional mapping (Figure 7).
We use an electrogram-based technique to target and eliminate electrical potentials and ensure continuity of ablation sets, with additional guidance by 3-dimensional cardiac mapping systems and intracardiac echocardiography. We also use contact force-sensing catheters to ensure catheter-tissue contact during ablation and to avoid excessive contact, which may enhance the safety of the procedure.
Energy: Hot or cold
Two types of energy can be used for ablation:
Radiofrequency energy (low voltage, high frequency—30 kHz to 1.5 mHz) is delivered to the endocardial surface via a point-source catheter. The radiofrequency energy produces controlled, focal thermal ablation.
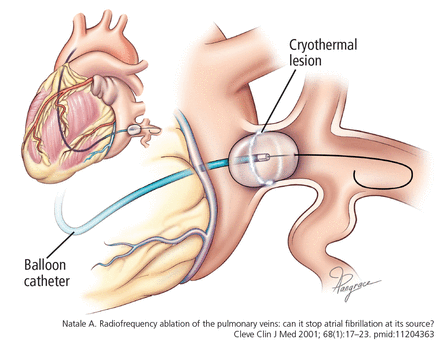
Cryothermal energy, ie, extreme cold, is delivered by a balloon catheter to create circumferential lesions around the pulmonary vein antrum (Figure 5).
A balloon catheter lodged in the ostium of one of the pulmonary veins to create a circumferential cryo-thermal lesion, electrically isolating the pulmonary vein.
In a randomized trial,25 these ablation technologies were shown to be equivalent for preventing recurrences of atrial fibrillation. We use both in our practice. The choice depends primarily on the planned ablation set, given that balloon cryoablation can achieve antral isolation of the pulmonary veins but allows little or no substrate modification.
Improved ablation technology
Intracardiac echocardiography, performed with an endovascular catheter in the right atrium, directly displays the interatrial septum, left atrium, pulmonary veins, ablation catheter, and catheter-tissue interface during ablation (Figure 6). It is used to guide trans-septal puncture, assess tissue-catheter contact during ablation, and monitor for complications. We also use it in balloon cryothermal ablation to ensure proper occlusion of the targeted pulmonary vein by Doppler assessment.
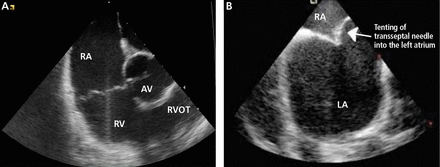
Intracardiac echocardiographic images. A, view with the probe located in the right atrium. B, view during transseptal puncture, routinely performed under intracardiac echocardiographic guidance. AV = aortic valve, LA = left atrium, RA = right atrium, RV = right ventricle, RVOT = right ventricular outflow tract.
Contact force-sensing catheters
Radio-frequency ablation catheters are now equipped with a pressure sensor at the tip that measures how hard the catheter is pressing on the heart wall.26,27 In our experience, this has improved the outcomes of ablation procedures, primarily in persistent atrial fibrillation.28
Three-dimensional cardiac mapping is now universally used for ablation of atrial fibrillation. It uses either electromagnetic data or impedance data to create a real-time 3-dimensional map of the heart (Figure 7) and to indicate the position of the ablation catheter. This technology significantly reduces the radiation dose to the patient, as well as the operator.
Three-dimensional voltage mapping of the left atrium. Top row, before ablation. Bottom row, after ablation. Voltage is color-coded: pink represents good voltage, red represents very low voltage, and other colors represent other points in the spectrum. LIPV = left inferior pulmonary vein, LSPV = left superior pulmonary vein, RIPV = right inferior pulmonary vein, RSPV = right superior pulmonary vein.
Complications of ablation
Although catheter ablation for atrial fibrillation is safe, it is still one of the most complex electrophysiologic procedures. Improvements in technology and techniques and accumulated experience over the past 15 years have made ablation safer, especially in tertiary care centers. But adverse outcomes are more frequent in low-volume centers.29
Minor procedural complications include pericarditis, complications at the site of vascular access, and anesthesia-related complications. While they do not affect the long-term outcome for the patient, they may increase hospital length of stay and cause temporary inconvenience.
Major complications include cardiac perforation and tamponade, periprocedural stroke, pulmonary vein stenosis, atrioesophageal fistula, phrenic nerve paralysis, major bleeding, myocardial infarction, and death. In a worldwide survey published in 2005, when atrial fibrillation ablation was still novel, the rate of major complications was 6%.30 By 2010, this had declined to 4.5%,31 and the rates of major complications may be significantly lower in more experienced centers.29 In our practice, in 2015, the rate of major complications was 1.3% (unpublished data).
Outcomes of catheter ablation
Clinical outcomes depend on many factors including the type of atrial fibrillation (paroxysmal vs nonparoxysmal), overall health of the atria (atrial size and scarring), patient age and comorbidities, and most importantly, the center’s and operator’s experience.
In randomized controlled trials comparing ablation and antiarrhythmic drug therapy, the efficacy of ablation in maintaining sinus rhythm has been in the range of 66% to 86% vs 16% to 22% for drug therapy,32,33 but these trials have been predominantly in middle-aged white men with paroxysmal atrial fibrillation. These trials also showed that catheter ablation reduced symptoms and improved quality of life. Ablation is less effective in persistent than in paroxysmal atrial fibrillation.34
In a long-term study from our group,14 660 (79.4%) of 831 patients who underwent ablation in 2005 were arrhythmia-free and not on antiarrhythmic drug therapy after a total of 1,019 ablations (an average of 1.2 ablations per patient) at a median of 55 months; 125 patients (15%, 41 with more than 1 ablation) continued to have atrial arrhythmia, controlled with drugs in 87 patients (69.6%). Only 38 patients (4.6%) continued to have drug-resistant atrial fibrillation and were treated with rate control with negative dromotropic agents.
Recent evidence
The largest randomized controlled trial of catheter ablation vs drug therapy for atrial fibrillation (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation [CABANA]) was completed recently, and the results were presented at a national meeting, although they have not yet been published in a peer-reviewed journal.35
A total of 2,204 patients with atrial fibrillation (42.4% paroxysmal, 47.3% persistent, and 10.3% long-standing persistent) were randomized to either ablation or drug therapy. Median follow-up was 4 years. The crossover rate was high—9.2% of those randomized to ablation did not undergo it, and 27.5% of those randomized to drug therapy underwent ablation.
The incidence of the primary end point (a composite of death, disabling stroke, serious bleeding, and cardiac arrest) was not significantly different between the 2 groups in the intention-to-treat analysis; however, given the high crossover rates, the as-treated and per-protocol analyses become important, and as-treated and per-protocol analyses revealed a significant benefit of ablation compared with drug therapy. The hazard ratio (HR) for the primary composite outcome was 0.67 (P = .006) on as-treated analysis and 0.73 (P = .05) on per-protocol analysis. The HR for all-cause mortality was 0.60 (P = .005) on as-treated analysis.
PERIPROCEDURAL CONSIDERATIONS
Periprocedural anticoagulation
The risk of thromboembolism is increased during, immediately following, and for several weeks to months after atrial fibrillation ablation.36,37
During the procedure, the risk is related to transseptal sheath placement, electrode catheters in the left atrium, and char formation on ablation catheters. These risks are mitigated with proper and careful sheath and catheter manipulation, maintenance of bubble-free irrigation through lines and sheaths, use of irrigated catheters, and initiation of heparin before transseptal access. Heparin is also infused during the procedure, with close monitoring of activated clotting time.
Postprocedurally, the transiently increased clotting risk could be due to damaged endothelium from the ablation itself and stunning of atrial tissue, which results in impaired contraction. Damaged endothelium improves as the tissue heals, and the stunning resolves by electrical reverse remodeling with sinus rhythm maintenance.
In view of these risks, the referring physician and electrophysiologist must pay careful attention to anticoagulation before and after ablation.
Before the procedure
It is safe to continue anticoagulation uninterrupted through the procedure.38,39 If the patient is on warfarin, we want the international normalized ratio to be in the therapeutic range when we perform atrial fibrillation ablation, and the patient takes his or her usual dose on the day of the procedure. If taking a direct oral anticoagulant, patients typically skip a dose the day before ablation and again on the morning of the procedure, and resume taking it immediately afterward while in the anesthesia recovery room.
During the procedure, we start heparin before transseptal puncture, adjust it to achieve an activated clotting time of 300 to 400 seconds, and keep it in this range as long as there are sheaths or catheters in the left atrium.
After the procedure
The current guide lines24 recommend that oral anticoagulation be continued without interruption for at least 2 months after the procedure, and in most cases indefinitely, depending on age and comorbidities. The decision to stop anticoagulation after 2 months is typically based on the stroke risk as assessed by the CHA2DS2-VASc score (www.chadsvasc.org) and not on the success of the ablation procedure.
ANTIARRHYTHMIC DRUGS AFTER THE PROCEDURE
Some patients actually experience more atrial fibrillation in the first weeks to months after the procedure. The mechanism in this setting may be different from that causing the arrhythmia in the first place. The causes of early recurrence of atrial arrhythmias include postablation inflammation, temporary autonomic imbalance, and delay of atrial radio frequency lesion formation.40,41 These arrhythmias may completely resolve as the ablation lesions heal and scars mature.
It has been hypothesized that short-term use of antiarrhythmic drugs after atrial fibrillation ablation is effective in preventing arrhythmias because it alters atrial electrophysiologic characteristics induced by the above transient factors. A recent systematic review of 6 clinical trials showed that short-term use of antiarrhythmic drugs reduces the risk of early arrhythmia recurrence but does not reduce recurrence in the long term.42
In terms of outcomes, any arrhythmias that occur in the first 3 months do not necessarily affect long-term success. This is referred to as the “blanking period.” However, generally speaking, it is preferable to maintain sinus rhythm during that time to avoid further anatomic or electrical left atrial adverse remodeling. In many situations, patients continue taking the same antiarrhythmic agent or start on antiarrhythmic therapy in the first few months after ablation.43,44
The mechanisms of late recurrence of atrial arrhythmias after ablation are thought to be different from those in early recurrence. Late recurrence has been ascribed to incomplete pulmonary vein isolation, recovery of pulmonary vein-left atrium connections, or recovery of any other lines of ablation created in the procedure.45,46 For late recurrence of atrial arrhythmia, studies and guidelines suggest that repeat ablation may be an option.24,47
PRACTICAL CONSIDERATIONS FOR PROCEDURAL PLANNING
Before the procedure, some electrophysiologists use cardiac computed tomography or magnetic resonance imaging to evaluate the pulmonary vein anatomy. This helps in planning and in selecting the appropriate tools for the procedure.
The patient is asked to fast on the day of the procedure. The procedure can take 3 to 6 hours, depending on the patient’s anatomy and the operator’s technique and experience. It can be performed with the patient under general anesthesia or conscious sedation. Currently, we use general anesthesia most of the time to maximize patient comfort.
After the procedure, our patients must stay in bed for 4 hours and stay overnight for observation. If no complications arise, they are discharged the next day.
- Copyright © 2018 The Cleveland Clinic Foundation. All Rights Reserved.