ABSTRACT
Functional tricuspid regurgitation (TR) develops secondary to annular dilation and leaflet tethering as a result of right ventricular remodeling. Invasive surgery for isolated TR is rarely performed due to high inpatient mortality. Transcatheter tricuspid valve intervention is an appealing solution but is challenging as crucial structures are closely related to the tricuspid valve, and intracardiac devices pose further challenges to device delivery and implantation.
Preprocedural multimodality imaging is essential to identify the appropriate device and to ensure procedural success.
Transcatheter tricuspid valve devices can be classified based on the mechanism of action.
To date, the MitraClip in the tricuspid position (TriClip) is the most utilized device for tricuspid valve repair. Modifications to the TriClip and Pascal device may improve applicability and outcomes.
Transcatheter tricuspid valve interventions appear to be associated with improvement in patient quality of life.
INTRODUCTION
Primary tricuspid regurgitation (TR) occurs as a result of an anatomically abnormal tricuspid valve. Trace or mild TR is common even in anatomically normal-looking valves and has no pathological implications. Certain etiologies such as rheumatic heart disease, prolapse, congenital disease (Ebstein anomaly), infective endocarditis, blunt wall trauma, endomyocardial biopsy-related trauma, and intra-annular right ventricular (RV) pacemaker or implantable cardiac defibrillator leads may result in more significant primary TR. However, it is important to note that close to 80% of TR cases are “functional” rather than primary.
Functional TR occurs secondary to annular dilation and leaflet tethering as a result of RV remodeling from either volume or pressure overload.1 The prevalence of functional TR in the United States is 1.6 million.2–4 Such RV remodeling and resulting TR frequently occur as a complication of left-sided valvular disease, with mitral valve disease being the most common culprit. Severe TR is associated with poor prognosis independent of age and biventricular function.5 Early studies in the 1960s suggested that treatment of left-sided valvular pathology (particularly mitral) may reverse pulmonary hypertension and therefore TR. However, more contemporary literature reveals that this process is gradual and often unpredictable.6
Currently, surgical techniques are the mainstay of treatment for progressive or severe functional TR. However, with the advent and success of transcatheter techniques for severe aortic stenosis and mitral regurgitation, there is newfound interest in creating safe and effective methods for minimally invasive management of functional TR.
ANATOMY, PATHOPHYSIOLOGY
Located between the right atrium and the RV, the tricuspid valve is slightly more apical than the mitral valve and consists of an annulus, leaflets, papillary muscles, and chordae tendinae (Figure 1).7
Schematic representation of the anatomy of the tricuspid annulus. The arrows represent annular dilation resulting in an increased anteroposterior diameter in functional tricuspid regurgitation.
The tricuspid valve is oriented at a 45-degree angle to the sagittal plane facing anterolaterally and inferiorly toward the left side. The annulus of the tricuspid valve is a nonplanar structure with a distinct bimodal or saddle-shaped pattern having 2 high points (oriented superiorly toward the right atrium) and 2 low points (oriented inferiorly toward the RV). The tricuspid annular area on 3-dimensional (3D) echocardiography has been estimated as 9.72 ± 2.08 cm2 in normal individuals. RV or annular dilation and tethering of the leaflets are the 2 main pathophysiologic mechanisms for the development of functional TR. While this is mostly secondary to left-sided disease, it may also be associated with isolated RV disease or pulmonary hypertension. The TR that develops through these mechanisms leads to further RV dilation and dysfunction and more tricuspid annular dilation and tethering, thereby progressively worsening the existing TR. This vicious cycle perpetuates TR and forms an integral part of the pathophysiology of severe TR. The increase in right-sided volumes and pressures impedes left ventricular relaxation and ejection, with resultant diastolic and sometimes systolic dysfunction.8,9
Anatomically, in patients with functional TR, the annulus is larger, flatter, and more circular thereby altering the saddle shape of a normal valve. The dilation of the tricuspid annulus in functional TR is not symmetric. It has been shown that the anteroposterior distance increases by approximately 80%, whereas the mediolateral distance increases by only 34%. This is because of a greater dilation along the free-wall aspect of the annulus (Figure 1).5,10,11
IMAGING GUIDELINES AND CHALLENGES
TR requires multimodality imaging with 2D transthoracic echocardiography (TTE), 3D TTE, computed tomography (CT), and magnetic resonance imaging (MRI). While TTE and transesophageal echocardiography (TEE) are excellent at visualizing the tricuspid valve leaflets, CT and MRI are superior for accurate assessment of the tricuspid annulus geometry (Figures 2 and 3).
(A–C) Transthoracic echocardiography (TTE) views and (D–F) transesophageal echocardiography (TEE) views of the tricuspid valve (TV). (A) TTE 4-chamber view: The septal leaflet is shown in red. The other leaflet (orange) could be the anterior leaflet (if probe is angled anteriorly and the aortic valve is brought into view, ie, 5-chamber view) or the posterior leaflet (if probe is angled posteriorly and coronary sinus brought into view). (B) TTE RV inflow view: The anterior leaflet is shown in blue. The other leaflet (in green) could be the posterior leaflet or the septal leaflet. (C) TTE 3D focused on the tricuspid valve. Three orthogonal planes centered on the TV are cross-referenced (inlets on the left), allowing easier identification of the different leaflets. (D) TEE 4-chamber view: As in the TTE 4-chamber view, the septal leaflet (in red) is identified, and the other leaflet (orange) could be either the anterior or posterior. (E) TEE RV inflow view: The posterior leaflet is shown in purple, the other leaflet (in grey) could be either the anterior or septal leaflet. (F) TEE 3D focused on the tricuspid valve. As in the 3D TTE, 3 orthogonal planes are cross-referenced. The different leaflets are easier to identify.
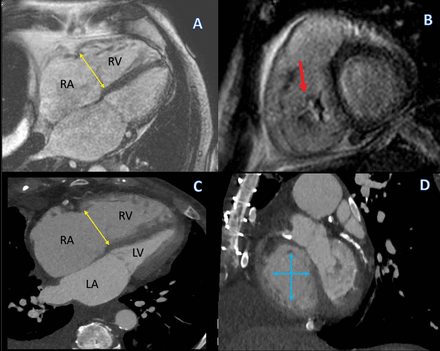
(A and B) Steady-state free precession magnetic resonance images in a 4-chamber and short-axis orientation, respectively. (A) Severely dilated tricuspid annulus (double arrow in yellow). The arrow in D points to a large area of central regurgitation (flow dephasing) caused by a lack of leaflet coaptation secondary to severe annular dilation. (C) Corresponding computed tomography image in a 4-chamber orientation also demonstrating an enlarged annulus (double arrow in yellow). The annular enlargement is better appreciated on the short-axis view (D, blue arrows).
The first step to imaging the tricuspid valve is invariably 2D TTE. TTE can distinguish the etiology of TR, quantify its severity, and determine the annular dimensions. Some argue that the traditional classification of TR has limitations and the definition of severe TR is broad, thus a new classification has been proposed: mild, moderate, severe, massive, and torrential.12 Better classification of TR can potentially help clinicians identify patients who would benefit from transcatheter valve therapies. Similarly, a staging system has also been proposed for functional TR (Table 1) based on annular dilation, RV function, remodeling, TR severity, leaflet coaptation, and right-sided heart failure.13
Staging of functional tricuspid regurgitation
Significant annular dilation on TTE is defined as diastolic diameter greater than 40 mm or greater than 21 mm/m2. Simultaneous assessment of all 3 leaflets can be challenging; thus, different projections are useful (long-axis RV inflow, short-axis at the aortic valve, apical 4-chamber view, and subcostal views).10 Despite this, the designation of individual leaflets of the tricuspid valve should be done with caution unless all 3 leaflets can be seen simultaneously. Real-time 3D TTE proves to be useful in situations where this is not possible and allows, through its ability to obtain a short-axis view of the tricuspid valve, simultaneous visualization of all 3 leaflets moving during the cardiac cycle. It also allows visualization of their commissures and attachment to the tricuspid annulus.8,9,14,15 3D TTE can also be used to assess RV volumes; however, wherever available, cardiac MRI is the gold standard for assessing RV function and volumes.16 The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend 3D TTE or MRI for evaluation of RV systolic function and RV volumes in patients with severe TR and suboptimal TTE (Class IIb, level of evidence C). CT offers in-depth assessment of various dimensions relevant to the tricuspid valve. This includes the dimensions of the RV, tricuspid valve annulus, and the annular distance from the RV apex. The venous anatomy and dimensions of the subclavian and axillary veins can guide access-site selection. The appropriate fluoroscopic angles for valve deployment can be derived from the CT.17 CT can also detect the course of the right coronary artery to the tricuspid annulus, and a course of 2 mm or less is considered high risk, which is a common finding in about 40% of patients with severe TR.18,19
MANAGEMENT OF FUNCTIONAL TR
Based on the 2014 ACC/AHA guidelines for valvular heart disease, grading of TR is done based on the central jet area, vena contracta width, continuous jet density and contour, and hepatic vein flow. Table 2 details the stages of TR based on these guidelines.
Echocardiographic grading of tricuspid regurgitation
The guidelines recommend the use of diuretics in severe TR and signs of right-sided heart failure (Class IIa, level of evidence C). Medical therapies to reduce elevated pulmonary artery pressures or pulmonary vascular resistance or both are also recommended in severe functional TR (Class IIb, level of evidence C, D). Table 3 displays a comparison of ACC/AHA guidelines (2014) and the European Society of Cardiology (2017) guidelines for surgical management of functional TR.1,20
Guidelines for the management of functional tricuspid regurgitation
Patient selection
Timely referral to a tertiary center is essential to achieve an optimal result after transcatheter tricuspid valve intervention. Functional TR is a common sequela of previous or current severe left-sided heart disease. The concept that surgical or percutaneous management of left-sided disease leads to spontaneous resolution of TR is a misconception. Concomitant treatment of TR and left-sided disease should always be considered during the index procedure. From the surgical literature, some of the predictors for recurrence of TR are tethering distance greater than 76 mm, mitral valve replacement, pulmonary artery pressure of over 90 mm Hg, severe RV impairment, left ventricular dysfunction and advanced left ventricular remodeling, intracardiac devices, and suture annuloplasty.21–26 Patients with severe pulmonary hypertension and RV dysfunction do not tolerate a precipitous reduction in TR and can develop acute afterload mismatch and decompensation.27–29 All the factors mentioned above should be taken into consideration and the severity of TR should not be the primary reason for referral. Multiple staging systems have been proposed recently based on annular dilation, RV function, remodeling, TR severity, leaflet coaptation, and right-sided heart failure.13,30,31 Stages 4 and 5 as listed in Table 1 are not suitable for percutaneous transcatheter tricuspid valve management.
Percutaneous tricuspid interventions: Devices and outcomes
Use of transcatheter approaches for the treatment of severe aortic stenosis and mitral regurgitation has led to growing interest in adopting these techniques for severe functional TR. However, challenges to the tricuspid edge-to-edge repair include the trileaflet nature of tricuspid valve, wide malcoaptation gaps, higher chordal density, and fragility of the leaflet and annular tissues. Additionally, the proximity to the conduction system, right coronary artery, and coronary sinus potentially predispose to procedural complications.32,33 However, despite these difficulties, there are several reasons to develop and advance novel methods of minimally invasive, percutaneous TR treatment. Medical treatment is restricted to the use of diuretics and can only partially address the symptoms,3 and TR is associated with poor prognosis both in isolated disease and when associated with left-sided pathology. It is also important to note that patients with TR may have less benefit from percutaneous procedures for mitral regurgitation.34,35
Several devices and strategies are being evaluated for the treatment of functional TR. These can be broadly classified by mechanism into leaflet approximation devices, annuloplasty devices, transcatheter tricuspid valve implantation, and caval valve implantation.
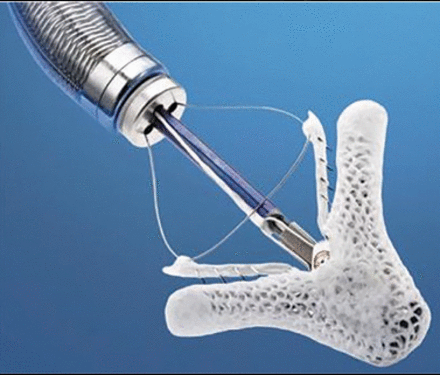
Leaflet approximation device. Sixty-four patients from 10 international centers were treated with the MitraClip (Abbott Vascular) for TR. Functional TR was present in 88% of patients. Results showed significant improvements in echocardiographic and functional parameters without any procedural adverse events with the use of the MitraClip device (Figure 4). It is interesting to note that patients who had a combined mitral and tricuspid MitraClip procedure did not have a significant increase in 6-minute walk distance at 30 days. However, there was a similar echocardiographic improvement in patients undergoing tricuspid clipping alone and those undergoing combined mitral and tricuspid clipping.36
MitraClip.
The multicenter international TriValve (Transcatheter Tricuspid Valve Therapies) registry reported 1-year outcomes after edge-to-edge repair in 249 patients with severe TR.37 Nearly 90% of the cohort had functional TR and over half the population underwent concomitant treatment of tricuspid and mitral regurgitation. The mean age of the cohort was 77 and the mean European System for Cardiac Operative Risk Evaluation II score was 6.4%. Procedural success was achieved in 77% of the patients (TR reduction to grade ≤ 2+). At 12 months in nearly 70% of the patients there was sustained improvement in TR severity and New York Heart Association functional class (≤ II). Death due to any cause was 20%. The risk factors for procedural failure were the absence of central or anteroseptal TR jet location, effective regurgitant orifice area greater than or equal to 0.70 cm2, tricuspid coaptation gap greater than 0.65 cm, and tenting area greater than 3.15 cm2.37
The TRILUMINATE trial was a single-arm study of 85 patients with severe functional TR.2 No periprocedural deaths, myocardial infarctions, or strokes occurred. At 6 months, all-cause mortality was reported in 5% (4 of 84) of patients and the rate of major bleeding was 11% (9 of 84). Single leaflet device attachment was seen in 7% of patients (5 of 72) and tricuspid valve stenosis was noted in 9% of patients (6 of 65).
The PASCAL system (Edwards Lifesciences) consists of a 10-mm central spacer that blocks the regurgitant orifice. It attaches to the leaflets with 2 paddles and was first used in an 82-year-old woman with secondary TR and advanced right-sided heart failure.38 Two devices were implanted and the patient was discharged at 72 hours. At 1-month follow-up there was a resolution of ascites, and the dose of diuretics was lowered, which was associated with improved quality of life and 6-minute walk distance. The Edwards CLASP TR EFS (NCT03745313) study is evaluating the safety and efficacy of the PASCAL system in patients with severe symptomatic functional or degenerative TR.
Annuloplasty systems. Annuloplasty devices recreate surgical techniques and address the primary pathophysiologic mechanism of TR, annular dilation. All annular-based devices require sufficient annular tissue for anchoring and may be prone to dehiscence if tissue quality is poor or excessive force is applied. Therefore, they may have limited effectiveness in patients with advanced RV disease and excessive tethering.39
The TriCinch system (4Tech Cardio Ltd.) is a percutaneous device designed for tricuspid remodeling, using a transfemoral fixation of a stainless-steel corkscrew into the anteroposterior tricuspid valve annulus. The corkscrew is connected through a Dacron band to a self-expanding Nitinol stent. By pulling the system towards the inferior vena cava, the anchoring corkscrew remodels the anteroposterior annulus, and the tension is maintained by fixation of the stent in the inferior vena cava. The first use of this device was reported in 2015 for the treatment of functional TR in a 72-year-old woman with right-sided heart failure and repeated decompensations. The patient reportedly had good functional status at 6-month follow-up.40 The TriCinch Coil System was also performed with deliberate creation of pneumopericardium to guarantee the appropriate advancement of the device in a woman age 81 with severe functional TR.41 The PREVENT trial (Percutaneous Treatment of Tricuspid Valve Regurgitation With the TriCinch System) reported a procedural success rate of 94% in 18 patients (≥ 1-grade reduction of TR).42 Two patients developed pericardial tamponade periprocedurally, and leaflet device detachment was noted in 4 patients. Significant improvement in 6-minute walk distance and quality of life was also noted. The safety and efficacy of the TriCinch System is currently being assessed in clinical trials (NCT03294200 and NCT03632967).
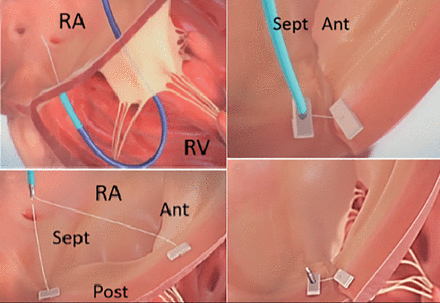
Another novel method of reducing functional TR is via plication of the tricuspid annulus using the TriAlign system (Mitralign Inc.) (Figure 5). This system consists of a deflectable guide catheter introduced using a transjugular approach to position a wire catheter on the ventricular side of the tricuspid annulus, and then to introduce an insulated radiofrequency wire across the annulus. Thereafter, a pledgeted suture is delivered (using a catheter over the radiofrequency wire) and the process is repeated to insert a second pledget. The sutures are then plicated, leaving behind a bicuspid tricuspid valve with reduced annular dimensions and a regurgitant orifice. Recently, 30-day results from the SCOUT trial (Percutaneous Tricuspid Valve Annuloplasty System for Symptomatic Chronic Functional Tricuspid Regurgitation) were published using this procedure. In a cohort of 15 patients, they showed that there was 80% success without the need for reintervention.43 The SCOUT II trial, an open-label nonrandomized clinical study, is currently recruiting patients with evidence of functional TR secondary to annular dilation (NCT03225612).
TriAlign system.
Ant = anterior; Post = posterior; RA = right atrium; RV = right ventricle; Sept = septal
Source: SCOUT study video from HeartValveSurgery.com
An exciting new development has been the Cardioband (ValtechCardio), which mimics open-heart annuloplasty for TR. It is a transcatheter annuloplasty system designed to implant a Dacron surgical-like adjustable band with a sutureless technique under echocardiographic and fluoroscopic guidance. The device is delivered via the transfemoral approach and is secured with 17 anchors on the atrial side of the anterior and tricuspid annulus. The first reported use in humans was published in early 2017 in a patient with functional TR and annular dilation who underwent the procedure successfully. The TRI-REPAIR study consisted of 30 patients undergoing Cardioband for moderate to severe functional TR.44 The average age of participants was 75 and most were women. Procedural success was 100%, with a substantial decrease in annular septolateral diameter, effective regurgitant orifice area, and vena contracta width at 6 months. Similar results were reported by the Cardioband TR EFS Investigators in 22 patients45 (NCT03382457). The cohort consisted of mostly women (77%) with a mean age of 78. Atrial fibrillation and flutter were common comorbidities, with a 96% prevalence rate, and the procedural success rate was 95%. Complications included 1 right-sided coronary artery constriction, and 7 patients suffered a major bleeding event. There was no cardiovascular mortality, myocardial infarction, or stroke.
Another device in the investigation phase for tricuspid repair is the minimally invasive annuloplasty (MIA) technology (Micro Interventional Devices, Inc.). Two patients in Lithuania received this device with no intraoperative complications, based on media reports. The Study of Transcatheter Tricuspid Annular Repair (STTAR) is a multicenter safety and performance study being conducted in Europe to evaluate the use of this device.46
A new device under development is the Millipede system (Millipede Inc.), which is an adjustable, semi-rigid ring attached to the annulus by rotational anchors positioned at defined intervals. The device has a zigzag appearance like the top of a crown, with the anchors at the lowest points and a collar around the hinge points at the crests. The annular reduction is then accomplished by repositioning the collars further down the crest, effectively reducing the distance between the anchors.47 The pledget-assisted suture tricuspid annuloplasty (PASTA) device mirrors the Hetzer double-orifice suture procedure.48 The first in human experience resulted in annular dehiscence of the device 2 days after its insertion and this device potentially should be not be used in large friable annuli.48
Transcatheter tricuspid valve implantation. The Gate valve (NaviGate Cardiac Structures Inc.) is an atrioventricular valved stent that has been developed for the treatment of TR (Figure 6). The first reported use in humans of this catheter-guided tricuspid atrioventricular valved stent was at Cleveland Clinic in a 64-year-old woman with multiple comorbidities including end-stage renal disease and multiple prior admissions for right-sided heart failure. The tricuspid annulus measured 50 mm × 40 mm on a focused 4D CT study. The patient tolerated the implantation well and was transferred to the ICU. Although she had a prolonged hospital course, she was discharged on postoperative day 29. The predischarge TTE for this patient showed moderate to severe RV dysfunction and mild to moderate TR, and paravalvular leak. The second patient was a 78-year-old man with significant cardiac history including coronary artery disease, previous myocardial infarction, atrial fibrillation, and 3 prior open-heart surgeries for coronary bypass, mitral valve repair, and 2 tricuspid valve repairs (annuloplasty ring 34 mm). He had a progressive decline in functional capacity due to right-sided heart failure that was refractory to medical treatment. He was discharged successfully on postoperative day 7, and predischarge TTE showed severe RV dysfunction and mild paravalvular TR. Both of these patients were deemed to be at prohibitive risk of open-heart surgery by a multi-disciplinary team.49
Gate valve.
Caval aortic valve implantation (CAVI). Heterotopic tricuspid valve implantation is a potential option in patients with severe TR and significant venous con gestion. The valve in this technique is inserted percutaneously into the inferior vena cava to protect the abdominal vasculature from elevated venous pressures and systolic backflow from severe TR. The upper valve segment protrudes into the right atrium, and the lower segment anchors to the inferior vena cava. The first reported experience with CAVI was reported by Lauten and colleagues in 2011.50 The valve implantation was successful, and the patient’s functional capacity and heart failure symptoms improved. Right atrial volume overload and right atrial ventricularization are potential demerits of CAVI.51
The Treatment of Severe Secondary Tricuspid Regurgitation in Patients With Advance Heart Failure With Caval Vein Implantation of the Edwards Sapien XT Valve (TRICAVAL) trial consisted of 28 patients with severe symptomatic TR that were randomized to optimal medical therapy or Edwards Sapien XT valve implantation (NCT02387697).52 There was no difference noted in functional end points (6-minute walk test, RV function, hospitalizations, and quality of life) across the intervention and control arms. TRICAVAL was cancelled due to safety concerns. The Heterotopic Implantation of the Edwards-Sapien Transcatheter Aortic Valve in the Inferior Vena Cava for the Treatment of Severe Tricuspid Regurgitation (HOVER)(54) trial is currently evaluating the feasibility of this device53 (NCT02339974).
Device-lead-induced TR
Patients with device-lead-induced TR are a special subgroup. The reported incidence of lead-induced TR is as high as 45%54,55 and is linked to a poor prognosis.56 It has been postulated that this is caused by bulky leads and apical lead placement and that the leads also alter the RV geometry resulting in TR.57,58 TR can also be a result of leaflet perforation during lead placement. Other mechanisms include lead impingement or adherence and lead entrapment in the subvalvular apparatus of the tricuspid valve. Imaging can be challenging, and the severity of lead-induced TR is often underestimated.59 Newer imaging tools like calculation of regurgitant fractions and volumes based on 3D data sets could potentially overcome this challenge. Percutaneous transcatheter tricuspid interventions for lead-induced TR have been investigated using various devices including MitraClip, FORMA, TriCinch, Trialign, Cardioband, NaviGate, and CAVI.54 There is a risk of lead damage after device implantation and potentially impedes future lead extraction in case of lead endocarditis.60
FUTURE TRIALS
Several clinical trials of new devices for TR are currently under way (Table 4). To date, clinical trials have been done in patients with advanced stages of functional TR and there is an absence of a standard definition of clinical and imaging outcomes. Ideal trial design should include patients without RV dysfunction, remodeling, and right-sided heart failure that are randomized to surgical management or percutaneous intervention. The lack of specific clinical symptoms relevant to tricuspid valve disease and heavy reliance on quantitative assessment of TR, annular dilation, and RV size and function as imaging end points pose significant challenges for device approval. Important exclusion criteria should include severe pulmonary hypertension, severe RV failure, and other significant comorbidities (frailty, advanced kidney disease, liver dysfunction, severe lung disease). Another consideration could be given to a prospective registry of concomitant percutaneous mitral and tricuspid intervention for severe functional mitral regurgitation. Long-term preservation of RV function and preventing TR progression after tricuspid intervention could be demonstrated in such a cohort.
Clinical trials investigating the use of new devices for tricuspid regurgitation
CONCLUSION
Functional TR is a common valvular heart disease that is often overlooked and is linked to a poor prognosis. There are numerous percutaneous and minimally invasive options under different stages of investigation. The published data so far on percutaneous therapies demonstrate promising results in the form of a reasonable reduction in TR along with substantial improvement in the quality of life. The transcatheter device technology is currently evolving for the tricuspid valve. The evidence base in this intervention is growing rapidly; however, it is far behind its aortic counterpart.61,62 To improve the existing technology, it is imperative to understand tricuspid valve anatomy using multimodality imaging to identify patients early and prevent irreversible RV failure. Patient selection based on anatomy for the appropriate device technology (ie, coaptation vs annuloplasty vs replacement) is imperative. Improved device technology best matched to patient factors is likely to increase the array of options available to patients for TR.
Footnotes
All authors reported no financial interests or relationships that pose a potential conflict of interest with this article.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.