ABSTRACT
Asthma is highly prevalent and sometimes deadly, especially in certain groups. The 2019 Global Initiative for Asthma (GINA) guidelines recommend that all asthma patients be treated with inhaled corticosteroids taken daily or as needed; this improves symptoms and outcomes, even in those with mild disease. Further, asthma management requires a stepwise approach, escalating and de-escalating treatment based on symptom control.
Asthma is more common and more severe in women, Black people, and families with low income.
As asthma progresses in severity, treatment should no longer be taken only as needed but rather daily. Dosages should be increased, and a long-acting muscarinic antagonist should be added.
Before escalating treatment, clinicians should ensure that the patient is correctly and consistently using the prescribed medications and that asthma triggers have been reduced as much as possible.
Asthma and chronic obstructive pulmonary disease often occur together in elderly patients and those who smoke, requiring aggressive treatment such as triple therapy with an inhaled corticosteroid, a long-acting beta-agonist, and a long-acting muscarinic antagonist.
The Global Initiative for Asthma (GINA) updated its management guide-lines in 2019, recommending for the first time that every patient be treated with an inhaled corticosteroid (ICS), taken as needed or daily. This contrasts with older guidelines that recommended short-acting beta-agonists (SABAs) as rescue medications for mild-intermittent asthma, without any inhaled corticosteroid use.
This article briefly reviews the epidemiology, pathophysiology, clinical presentation, and diagnosis of asthma. Then, using case studies, we outline how to manage patients with mild, moderate, and severe asthma based on the GINA 2019 guidelines, as well as how to manage patients who have combined asthma and chronic obstructive pulmonary disease (COPD).
ASTHMA IS COMMON, ESPECIALLY IN CERTAIN GROUPS
Asthma affects nearly 25 million people in the United States, about 7.7% of the population.1 But it affects certain subgroups disproportionately, as follows:1
Women (9.8%) more than men (5.5%)
Non-Hispanic Black people (9.6%) more than non-Hispanic White people (8.2%), and Hispanic people (6.0%)
People in families with low incomes (< 100% poverty level; 10.8%) more than those with high incomes (> 450% poverty level; 6.5%).
Death rates reflect and sometimes amplify disparities in prevalence. In 2018, more than 3,400 asthma deaths were reported, with rates of 21.8 per 1 million in Black people, 9.5 per 1 million in White people, and 6.3 per 1 million in Hispanic people. Women died at the rate of 15.3 per 1 million and men at 10.2 per 1 million.1
ICS—inhaled corticosteroid
LABA—long-acting beta-agonist
LAMA—long-acting muscarinic antagonist
LTRA—leukotriene receptor antagonist
SABA—short-acting beta-agonist
Healthcare utilization by patients with asthma is high. In 2016, emergency department visits with asthma as the first-listed diagnosis occurred at the rate of 50.3 per 10,000 adults, and hospitalizations occurred at 4.4 per 10,000 adults.1
In 2018, 43% of adults with asthma reported having had at least 1 attack in the previous year.1
PATHOPHYSIOLOGY AND CLINICAL MANIFESTATIONS
The underlying pathophysiology of asthma is chronic airway inflammation, resulting in bronchoconstriction, airway wall thickening, and increased mucus production.2
Asthma can develop at any age, but most often in childhood. It is characterized by recurrent episodic respiratory symptoms such as wheezing, shortness of breath, chest tightness, and cough. Manifestations vary over time in duration, frequency, and intensity, so a patient’s physical examination may be normal at the time of presentation. Suggestive findings include expiratory wheezing, pale and swollen nasal mucosa, nasal polyps, and atopic dermatitis.
Typical triggers include respiratory infections, allergens, weather changes, poor air quality, tobacco smoke, exercise, stress, and laughing.2 A family or personal history of allergic disease supports the diagnosis. The diagnosis of asthma requires a compatible history as well as evidence of a variable and significantly reversible expiratory airflow limitation, measured by spirometry or peak flow (Table 1).2
Signs of airflow limitation variability
UPDATED MANAGEMENT GUIDELINES
When asthma is effectively treated, patients can achieve good symptom control, have productive, physically active lives, and exhibit normal or nearly normal lung function.2 All patients should be assessed and counseled on modifiable risk factors and triggers, such as smoking, medications (eg, nonselective beta-blockers), allergens, rhinosinusitis, obesity, gastroesophageal reflux disease, sleep-disordered breathing, depression, and anxiety.2 Patients should then be managed in a stepwise approach, escalating or de-escalating treatment based on symptom control, and subsequently reviewing treatment response.2
If a particular regimen does not control a patient’s asthma, before stepping up the treatment, one should reassess the patient’s adherence to the prescribed medications (and whether he or she can afford them), inhaler technique, modifiable risk factors, triggers, and comorbidities.2 Indicators of poor symptom control include frequent symptoms or reliever inhaler use, activity limited by asthma, and night-waking due to asthma. Stepped-up therapy can be short-term (1–2 weeks) when a trigger is temporarily present, such as during a respiratory infection, or indefinite if no apparent trigger is identified.2
According to the 2019 GINA guidelines, all patients should be treated with an ICS, taken either daily or driven by symptoms. Multiple randomized controlled trials and observational studies have found that this treatment improves symptoms, reduces decline in lung function, and reduces the risk of serious exacerbations, hospitalizations, and mortality, even in patients with mild asthma.1–4 This recommendation is a change from previous guidelines, which relied on SABAs for rescue for mild-intermittent asthma. ICSs address the underlying inflammatory process, while SABAs do not. Increased use of SABAs, which can signal worsening of asthma, is also associated with higher exacerbation risk.5,6
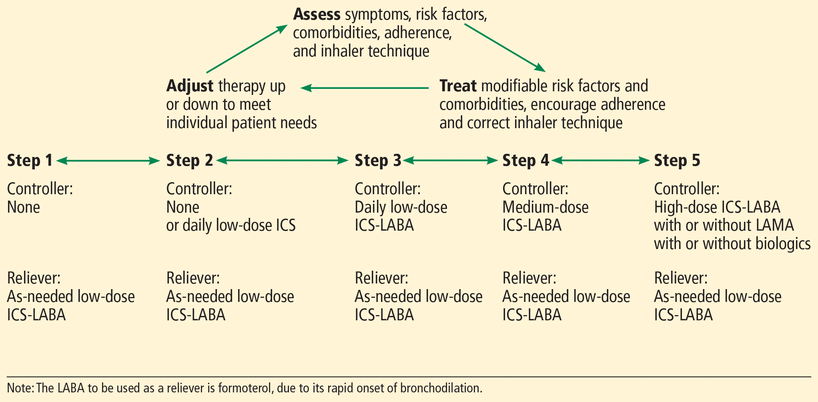
Management of mild, moderate, and severe asthma is summarized in Figure 1 and detailed in the cases below.
Stepwise approach to asthma management.
Based on Global Initiative for Asthma 2019 asthma management guidelines, reference 2.
CASE 1. A WOMAN WITH MILD ASTHMA
A 62-year-old woman presents to her doctor’s office for routine asthma follow-up. She was diagnosed with asthma 3 years ago and was initially prescribed a daily medium dose of an ICS plus a long-acting beta-agonist (LABA) inhaler for symptom control. Currently, she has been off maintenance inhaler therapy for more than a year and has not had an exacerbation in 2 years. She is symptom-free and on no medications. She has a short-acting beta-agonist (SABA) rescue inhaler but has not needed to use it in many months. Her comorbidities include obesity and uncontrolled gastroesophageal reflux disease. She received an influenza shot 2 weeks ago.
GINA 2019 recommends the following steps for managing mild asthma.
Step 1. Patients with symptoms occurring less than twice a month and who have no risk factors for exacerbation such as major environmental exposure, socioeconomic problem, or severely decreased lung function should be managed with either of the following regimens (level of evidence B—limited data including small randomized controlled trials and meta-analyses):
An ICS plus LABA combination (eg, budesonide-formoterol) in low doses as needed
An ICS and an SABA in low doses, to be used together as needed.
The former is recommended as an alternative to traditional reliever therapy with SABAs, but cost is often a barrier (the list price is $300–$346 for a 30-day supply, depending on dosage). Physicians should consider the cost when determining the treatment plan. Formoterol is the only LABA that is recommended to be used as a reliever, owing to its rapid bronchodilator action.
Step 2. Patients with symptoms occurring twice a month or more should be managed with either of the following regimens (level of evidence A—ample data based on appropriate studies):
An ICS plus LABA combination in low doses, as needed
An ICS in low doses daily, plus either one of these for rescue: a low-dose ICS-LABA or an SABA as needed.
Outcomes are similar with either the daily or as-needed strategy for mild disease, so patient preference should be considered, as well as the likelihood of adherence to daily treatment. Compared with patients with mild asthma who were treated with as-needed SABA monotherapy, those treated with daily low-dose ICS had half as many severe exacerbations in a study by Reddel et al,3 while those receiving as-needed low-dose ICS-LABA treatment had a 64% reduction in a study by O’Byrne et al.4 Other studies showed as-needed low-dose ICS-LABA therapy to be noninferior to daily ICS use for reducing severe exacerbations4,7 and exercise-induced bronchoconstriction.8 As-needed ICS-LABA treatment was, however, inferior to daily ICS therapy for symptom control.4,7
The clinician can also consider adding a leukotriene receptor antagonist (LTRA).
Case conclusion. The patient has controlled mild intermittent asthma. She is prescribed a low-dose ICS-LABA inhaler to use as needed, driven by symptoms. As obesity and gastroesophageal reflux disease can exacerbate asthma, she is encouraged to lose weight and is prescribed a proton-pump inhibitor. She is given a pneumococcal vaccination.
CASE 2. A MAN WITH MODERATE ASTHMA
A 51-year-old man presents to a physician’s office to establish care. He was diagnosed with asthma and hospitalized at a very young age. His asthma became mild after high school, and he has been off controller therapy for decades. A year ago, he began noticing chest tightness during exercise and recently has had to use his rescue inhaler on a daily basis. His asthma symptoms are triggered by stress, exposure to domestic animals, cold weather, exercise, and chest colds. He also has environmental, mold, and dust allergies. He has not had an exacerbation requiring prednisone since his youth and does not currently have any nighttime symptoms.
A few months ago he was started on low-dose ICS twice daily and LTRA therapy. Besides asthma, he has sleep apnea and uses continuous positive airway pressure most nights.
GINA 2019 recommends the following steps for managing moderate asthma.
Step 3. Patients who have symptoms present most days or who are waking up due to asthma at least once a week should be managed with the following regimen (level of evidence A):
Daily low-dose ICS-LABA combination, plus as-needed combined low-dose ICS-LABA or a SABA.
The first option uses ICS-LABA as controller and reliever.
For asthma that is uncontrolled on daily low-dose ICS, daily low-dose ICS-LABA leads to a 20% reduction in exacerbations and better lung function.2 For patients with at least 1 exacerbation in the previous year, maintenance and reliever treatment with low-dose ICS-LABA is more effective than maintenance ICS-LABA with as-needed SABA in reducing severe exacerbations, with similar symptom control.2
Another option for patients with uncontrolled symptoms on daily low-dose ICS is to increase it to a medium dose, but this is less effective than adding a daily LABA (level of evidence A).
The clinician may also consider an LTRA for these patients (level of evidence A).
Step 4. For patients with persistent symptoms despite adherence to step 3 therapy:
Manage with daily medium-dose ICS-LABA plus as-needed SABA (level of evidence B)
Consider daily high-dose ICS, LTRA, and long-acting muscarinic antagonist (LAMA).
If asthma remains uncontrolled, specialty referral should be considered.
Case conclusion. The patient has uncontrolled moderate asthma. His maintenance inhaler is switched from low-dose ICS to medium-dose ICS-LABA, and he should continue LTRA therapy. He is encouraged to use continuous positive airway pressure every night rather than most nights, remove animals from the home, use allergen-impermeable bedding covers, wash bedding weekly, clean moldy surfaces with bleach, and fix water leaks in the home as part of a comprehensive asthma treatment plan.
CASE 3. AN ELDERLY WOMAN WITH SEVERE ASTHMA
A 77-year-old woman presented to her doctor’s office for asthma monitoring. She was diagnosed with asthma in her 30s. Currently, her maintenance regimen is high-dose ICS-LABA and LTRA therapy. She reports adhering to her medications and demonstrates proper inhaler technique in the office. However, she has asthma symptoms daily and awakens because of asthma about twice a week. She was treated for an exacerbation 3 months ago. She reports smoking 4 to 10 cigarettes a day and having severe anxiety and depression.
GINA 2019 recommends the following steps for severe uncontrolled asthma.
Step 5. For patients whose asthma remains uncontrolled despite adherence to high-dose ICS-LABA and LTRA treatment, consider adding LAMA maintenance therapy. Specialty referral is strongly recommended. Patients should be evaluated for biologic therapy, ie, a targeted controller therapy that is prescribed by asthma specialists.
Case conclusion.The patient has uncontrolled severe asthma. Daily LAMA therapy is added to her regimen, and she is referred to a pulmonologist. As part of her comprehensive asthma management plan, smoking cessation is strongly encouraged, and a selective serotonin reuptake inhibitor is started. She is counseled that symptoms of anxiety and depression are associated with worse asthma symptom control, medication adherence, and asthma-related quality of life.2
ASTHMA-COPD OVERLAP SYNDROME
Asthma-COPD overlap syndrome is common, particularly in elderly patients and those who smoke.2,9 It is characterized by persistent airflow limitation on peak flow or spirometry, and diagnoses or features of both asthma and COPD (Table 2).2,9 It is regarded not as a single entity, but as a syndrome that includes several forms of airway disease caused by a range of poorly understood mechanisms.2,9
Asthma, COPD, and overlap syndrome
The overlap syndrome poses special challenges. Patients experience frequent exacerbations and tend to have poor quality of life.2,9 Their lung function declines more rapidly, their symptoms are more refractory to treatment, their mortality rate is higher, and they use disproportionately more healthcare resources than patients with either asthma or COPD alone.2,9,10
The exact prevalence of asthma-COPD overlap syndrome is difficult to estimate because of its heterogeneous nature, but has been reported to be between 1.1% and 4.5% in general population studies, and up to 27% and 33% in patients with asthma and COPD, respectively.9
Data are sparse on how to treat patients with overlap syndrome, as they are often excluded from clinical trials.2,9 More research is needed to elucidate underlying mechanisms contributing to the syndrome and to support the development of specific interventions to prevent and manage it.2,9
GINA recommends treating asthma-COPD overlap syndrome with low- or medium-dose ICS and adding an LABA or LAMA, or both, as needed to control symptoms.2 This recommendation emphasizes the importance of ICS in patients with asthma features. It is reasonable for patients with refractory symptoms to be treated with triple therapy (an ICS plus an LABA plus an LAMA).9 In a very small study, Ishiura et al10 found improved lung function in patients with asthma-COPD overlap syndrome when an LAMA was added to combined ICS and LABA. Biologics, phosphodiesterase-4 inhibitors, and macrolides may also have a role in treatment, but more research is needed.9 Current recommendations are based mostly on expert opinion and not outcome data.9
As in patients with asthma alone, risk factors and comorbidities should always be addressed and treated, and medication adherence should be monitored. Patients should be encouraged to exercise regularly, attend pulmonary rehabilitation, use oxygen if indicated, and receive proper vaccinations. Although initial recognition and treatment of asthma-COPD overlap syndrome may occur in primary care, specialty referral for confirmatory investigation is encouraged.2,9
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.