ABSTRACT
Aneurysm of the thoracic aorta is less common than in the abdominal aorta, but it is clinically important because of the risk of rupture and death. Cases are often found incidentally. Indications for surgical or endovascular repair are based on aneurysm location and risk factors for rupture such as aneurysm size, rate of growth, and associated conditions, while medical management is also important. Surveillance with various imaging tests is critical before and after intervention to guide treatment.
Patients with bicuspid aortic valve or genetic syndromes such as Marfan syndrome are at higher risk, with lower thresholds for surgical intervention, but account for only a minority of cases.
Although echocardiography has some roles in screening and monitoring the aortic root and ascending aorta, computed tomography and magnetic resonance imaging are necessary for the complete assessment of the thoracic aorta and are often necessary for surveillance.
Guidelines from several professional societies are available regarding surveillance and indications for intervention.
Patients with thoracic aortic aneurysm require multidisciplinary care, including a cardiologist and possibly a cardiovascular surgeon and genetic counselor.
Medical care includes traditional cardiovascular risk factor management. Beta-blockers are often used to control blood pressure but should be used with caution in those with acute aortic valve regurgitation.
Aneurysm of the thoracic aorta, renal artery, or splenic artery is often detected incidentally but can present acutely with dissection or rupture, with a high risk of death or morbidities. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are key to characterizing the aneurysm and the rest of the vasculature, while ultrasonography or echocardiography assist in assessment and surveillance, and catheter angiography is the gold standard for renal and splenic aneurysm.
The need for prophylactic intervention is based on aneurysm size, location, growth, and other associated conditions and risk factors in the individual patient. Management strategies include surgery, which is mandatory in the acute setting and in cases of challenging anatomy, and endovascular techniques. Regular imaging surveillance is critical after diagnosis and after aneurysm interventions.
In this, the first of 2 articles, we discuss thoracic aortic aneurysm (TAA); in the second article, we will discuss renal artery and splenic artery aneurysm.
WHAT IS THE CLINICAL IMPORTANCE OF TAA?
TAA is clinically important because of the risk of devastating complications—acute aortic syndromes such as aortic dissection and rupture.1,2
Type A aortic dissection (ie, originating in the ascending aorta) is a fatal condition with dismal in-hospital mortality rates of 57% without emergency surgery and 17% to 25% with emergency surgery in national and international registries despite advances in management.3,4 The mortality rate is much lower but still significant in expert aortic centers of excellence, such as the 4% to 7% reported by Cleveland Clinic.5 The incidence of combined TAA and aortic dissection has been reported to be 6 to 13 per 100,000 per year,6–8 although this would underestimate clinically silent TAA.3
There are no effective preventive strategies for TAA to date; thus, early detection, surveillance, and treatment are critical to improving outcomes. Guidelines are available.1,2,9
WHO IS AT RISK?
Risk factors for TAA (Table 1) are abundant in modern society and include older age, male sex, hypertension, smoking, and atherosclerosis. No wonder, then, that the incidence of TAA and the number of surgical repairs are increasing.2,10
Thoracic aortic aneurysm: Risk factors, associations, and causes
Genetic conditions associated with TAA such as Marfan syndrome are less common but nevertheless important because the prognosis and management are different.1,2,9 Some risk factors or conditions increase wall stress, while others increase medial degeneration.10 Although only 5% of cases of TAA are associated with genetic syndromes, another 20% are in patients who have a family history of TAA, which has important implications for assessment, management, and counselling.11 And many cases are idiopathic, lacking obvious causes or risk factors.
HOW IS TAA DISCOVERED?
Most cases of TAA are asymptomatic and are discovered either incidentally on imaging or as part of dedicated screening for those at risk.1 That said, possible symptoms include chest, abdominal, or back pain, dyspnea, cough, dysphagia, hoarseness, claudication, and cerebrovascular events.
The clinical history should be directed at symptoms, risk factors, and family history.
Physical examination should focus on the cardiac, neurologic, and peripheral vascular systems and should include blood pressure (and how it differs in different limbs), pulses, murmurs, and bruits, and other signs specific to associated conditions.1
Basic investigations that can detect possible abnormalities associated with TAA include electrocardiography (showing ischemic changes or myocardial hypertrophy), chest radiography (showing a widened mediastinum or prominent aortic shadow), and blood tests, including complete blood cell count, metabolic profile, and markers of inflammation, coagulation, and myocardial injury, many of which help in the differential diagnosis of TAA vs acute aortic syndromes.1,9
WHAT IS A NORMAL-SIZE AORTA?
Although aneurysm is generally defined as an increase of more than 50% of the normal arterial diameter, cardiac imaging guidelines have clear dimension thresholds for different severities of TAA dilation.9,10
The aorta is larger in men and in larger people generally, and therefore sex and body size are taken into account when determining the normal ranges and severity thresholds.9 The aorta also tends to increase in size with age. The upper limit of normal for aortic dimensions is 2 standard deviations above the mean diameter in a population of healthy adults.
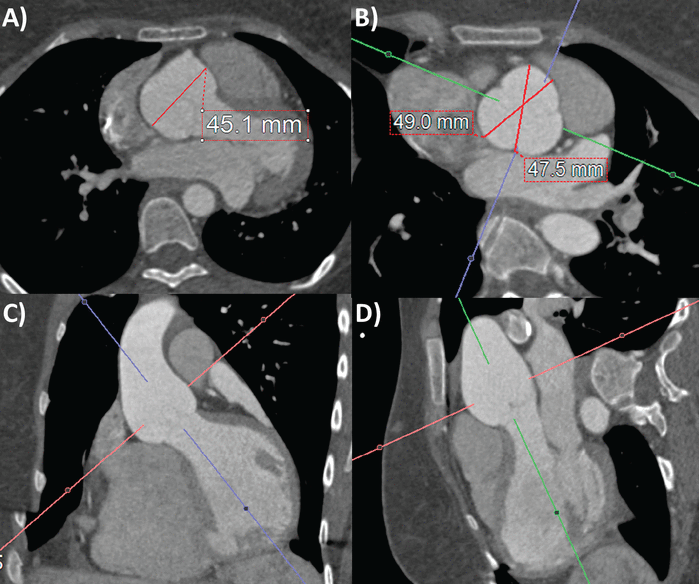
Aortic dimensions are measured at right angles to the direction of blood flow. On echocardiography, the standardized aortic measurements are taken in the end-diastolic frame and from leading edge to leading edge for reproducibility. On CTA and MRA, measurements are from inner edge to inner edge, from aortic sinus to sinus, or from sinus to commissure (often about 2 mm smaller than from sinus to sinus; Figure 1).12,13 The full thoracic aortic study should include measurement of all segments: aortic sinus; sinotubular junction; proximal, mid, and distal ascending aorta; aortic arch; and descending aorta, as well as the maximal dimensions, branch involvement, and surgical anastomoses.9 The aortic walls should be examined for calcification, throm-bus, dissection, hematoma, and infection.
Computed tomography angiography aortic root measurements on (A) axial source image and (B–D) 3-dimensional multiplanar reconstruction (3-D-MPR) double-oblique planes. Note that without 3-D-MPR, the aortic root size is underestimated (A). Also note that sinus-commissure measurements are often slightly less than sinus-sinus measurements in (B).
WHAT IMAGING MODALITIES ARE USED?
Aortic imaging remains central to TAA diagnosis and surveillance.1,2,9
Three-dimensional multiplanar reconstruction software for CTA and MRA has revolutionized measurement of the aorta, reconstructing source images into double-oblique planes to ensure measurements are taken perpendicular to the lumen (Figure 1).1,2,9
Echocardiographic aortic root measurement has the strongest evidence base for guiding intervention, and its thresholds have been extrapolated to other modalities and aortic locations. Clinicians need to be aware of these concepts and limitations to select the best imaging modality, perform measurements, and interpret the results. Table 2 lists the uses and limitations of 5 imaging modalities for TAA, modified from American Society of Echocardiography guidelines.9
Imaging options for assessing thoracic aortic aneurysm
Transthoracic echocardiography (TTE) has the advantages of portability, accessibility, and low cost. The operator should interrogate the aortic root and ascending aorta in the parasternal long-axis views, parts of the arch and descending thoracic aorta in the suprasternal view, and a segment of the abdominal aorta in the subcostal view.1,9
Transesophageal echocardiography (TEE) has a limited role in the primary assessment of TAA unless concurrent structural cardiac disease is suspected. It can visualize a greater extent of the thoracic aorta than TTE and with superior spatial resolution, including with 3-dimensional techniques. It can also be used for intraoperative evaluation as well as a contrast-free imaging option for diagnosing acute aortic syndromes.9 The aortic root and ascending aorta can be visualized in the midtransesophageal long-axis view at 100 to 140 degrees; the aortic valve and root in the short-axis view at 45 to 60 degrees; and the descending thoracic aorta up close at 0 degrees in the short-axis view and 90 degrees in the long-axis view, where atheroma and dissection flaps can be visualized up to the aortic arch with probe withdrawal.1,14
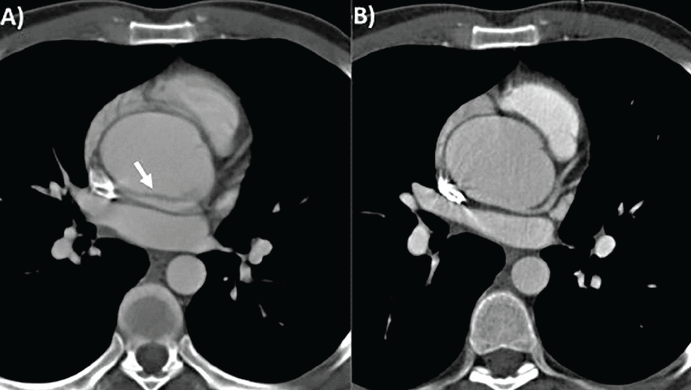
CTA is the recommended first-line imaging for assessing TAA, having high spatial resolution and a short scan time (3–4 seconds for the thoracic aorta, < 10 seconds for thoracoabdominal and iliofemoral vessels), enabling assessment of all segments and walls of the thoracic aorta with a 3-D dataset. Radiation and contrast use are limitations. Electrocardiographic gating of CTA is recommended to reduce motion artifacts (Figure 2).
Computed tomography of thoracic aortic aneurysm without (A) and with (B) electrocardiographic gating. Note that the motion artifact indicated by the white arrow in (A) is not seen in (B).
Noncontrast CT of the aorta may add value if assessing for intramural hematoma or vascular calcification, or if contrast is contraindicated.15
MRA also provides a high-resolution 3-D dataset for aortic assessment without the use of radiation, but has longer scan time, higher cost, and lower availability than echocardiography and CT, and so it is a second-line modality.9 Relevant magnetic resonance techniques include contrast-enhanced MRA, cine bright-blood sequences such as steady-state free precession and black-blood spin-echo sequences with or without inversion recovery. MRA can further assess aortic physiology, for example, measuring flow by phase-contrast velocity-encoded imaging, aortic stiffness and elasticity, and shear stress.3,16
Both CTA and MRA can also assess for other cardiac and thoracic diseases. CTA or MRA should be performed in every patient diagnosed with TAA to confirm the maximal dimensions and assess the entire length of the aorta.1,2,9
Other methods for aortic imaging include invasive aortography with fluoroscopy, positron-emission tomography, and intravascular ultrasonography, although they are never used solely for assessing TAA.1
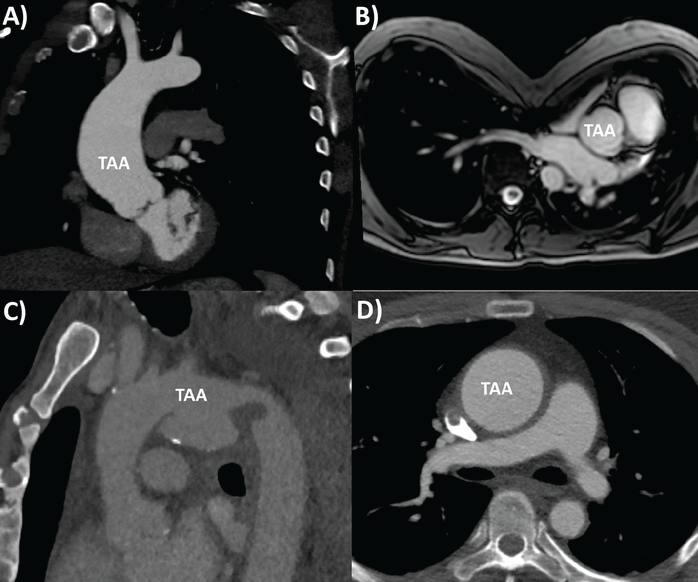
Examples of TAA pathologies are shown in Figure 3.
Range of thoracic aortic aneurysm (TAA) pathologies: (A) bicuspid aortic valve aortopathy on computed tomography (CT), (B) Marfan syndrome with pectus excavatum on magnetic resonance imaging, (C) mycotic aortic arch aneurysm on CT, (D) Takayasu arteritis on CT, with thickened, inflamed aortic wall.
WHEN SHOULD TAA BE FIXED?
Table 3 summarizes the American 2010 and European 2014 guidelines and our recommendations on indications for TAA repair.1,2 The main determinants include aneurysm dimensions, rate of expansion, and associated conditions. The patient’s overall estimated risk of acute aortic syndrome also needs to be balanced with the hospital’s expertise and procedural risks for TAA repair. Surgical evaluation is necessary when there are symptoms thought to be related to the TAA, irrespective of other factors.2
Indications for prophylactic intervention for thoracic aortic aneurysm
TAAs grow by 0.7 to 1.9 mm per year in undilated aortas, but growth can be faster in patients with a dilated aorta or associated conditions.17
TAA size is the strongest predictor of acute aortic syndromes.18 In patients who have no other conditions, the guidelines recommend surgery when the aortic root, ascending aorta, or aortic arch reaches 5.5 cm and when the descending aorta reaches 6.0 cm (≥ 5.5 cm with endovascular stenting).1,2 This is based on a sharp rise in the risk of aortic dissection when the ascending aorta reaches 6 cm and the descending aorta reaches 7 cm.17
Factors that lower the threshold include associated conditions, faster rate of growth (measured by the same modality and exceeding the margin of error of 3–5 mm/year), and the need for adjacent aneurysm or aortic valve surgery.1,2
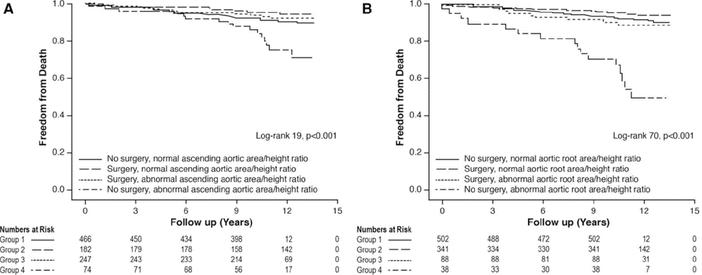
The American guidelines further emphasize measuring the maximal TAA cross-sectional area. If the maximal TAA cross-sectional area (in cm2) divided by height (in meters) is greater than 10, this would be another indication for intervention.2 This threshold was derived from studies from Cleveland Clinic originally applied to patients with bicuspid aortic valves and Marfan syndrome,19,20 and more recently in all TAA patients,21 with major prognostic implications (Figure 4).
Cross-sectional area-to-height ratio and management-stratification Kaplan-Meier survival curves for (A) aortic root and (B) ascending aorta in 969 consecutive patients with bicuspid aortic valve with proximal aorta diameter ≥ 4 cm, who underwent gated contrast-enhanced thoracic computed tomography or magnetic resonance angiography. Note the worse outcomes for those with aortic root area-to-height ratio > 10 cm2/m, in whom surgery makes a big difference in survival.
Reprinted from Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with bicuspid aortic valve and a dilated ascending aorta. Circ Cardiovasc Imaging 2017; 10(6):e006249. doi:10.1161/CIRCIMAGING.116.00624
Lower thresholds in associated conditions
Lower thresholds for intervention are recommended when patients have associated conditions that increase the risk of dissection at smaller dimensions and increase the rate of growth.1,2
Bicuspid aortic valve. Recent guidelines have shifted the thresholds for intervention back up to ≥ 5.5 cm, or ≥ 5.0 cm with risk factors for patients with bicuspid aortic valves, which occur in 1% to 2% of the population.1,22 (Previously, the threshold was 4.5 cm or greater.) These patients have a risk of aortic dissection up to 8 times higher than that of the general population.23 A Cleveland Clinic study found the risk of aortic dissection in bicuspid aortic valve patients to be elevated at 4.7 to 5.3 cm, but the risk further accelerates beyond 5.3 cm, so a 5.0-cm threshold for intervention rather than a higher one may indeed be preferred in these patients.24
Marfan syndrome. The threshold for intervention is 4.5 to 5.0 cm, depending on risk factors.1
Loeys-Dietz syndrome. There are mixed views for the threshold of intervention, ie, whether it should be the same as in Marfan syndrome or even lower.1,2,25
Turner syndrome is associated with short stature and greater risk of rupture for the same aorta size, so indexed measurements are preferred.26 It is also associated with bicuspid aortic valve and aortic coarctation, so concurrent cardiovascular surgery is often required.
Ehlers-Danlos syndrome is associated with tissue fragility, making surgery challenging. Therefore, surgery remains controversial in this condition, and most patients are conservatively managed.27
HOW SHOULD TAA BE MONITORED?
Patients with TAA should be referred to a cardiologist (and a surgeon, if approaching or exceeding surgical criteria) for optimal decision-making in surveillance and management.
The first thing to consider is the imaging modality to use. Table 4 summarizes the guidelines and our recommendations for TAA surveillance, using TTE, CTA, and MRA.1–3
Recommendations for measurement and surveillance of thoracic aortic aneurysms
CTA or MRA is useful at baseline to image the entire aorta and check agreement with TTE measurements. If TTE measurements have close agreement with CTA or MRA, then TTE can be used for regular monitoring, although CTA or MRA should still be performed, though less often, for monitoring segments of the aorta not visible on TTE and checking TTE accuracy over time.
If there is poor agreement between TTE and CTA or MRA measurements, or poor visualization of the aorta with TTE, then CTA or MRA should be used instead for regular monitoring. The latter is preferred to avoid radiation exposure, but the former may be necessary if MRA is contraindicated, eg, because of a cardiac device or claustrophobia.3 Accurate and reproducible measurements are critical in surveillance, especially when nearing the threshold for intervention.
Once the modality is established, timing of surveillance and guideline recommendations depend on aortic dimensions and growth and presence of associated conditions.1,2,9 In the absence of conditions associated with TAA, the recommendation is routine surveillance at the discretion of the clinician, based on individual risk. On the other hand, an early follow-up scan (6 months after initial TAA diagnosis) is recommended to assess for growth of the aneurysm in patients who have genetic conditions, and annually thereafter if measurements have been stable or more frequently if there is accelerated growth.
The measurements recommended may also differ by condition, such as comparing to normalized values by age, sex, and body surface area and using Z scores in those with Marfan syndrome and indexing to body surface area in those with Turner syndrome.9 No specific recommendations for TAA surveillance and intervention for Ehlers-Danlos syndrome have been made because there is no evidence that intervening is beneficial.1,2,9
DO DRUGS SLOW THE RATE OF TAA EXPANSION?
TAA patients should be referred to a cardiologist to provide guideline-based medical management of the aorta, and to a cardiac surgeon when nearing a threshold for intervention.1,2
Blood pressure control is the cornerstone of medical management of TAA, as it makes pathophysiologic sense to reduce aortic wall shear stress and expansion. However, many recommendations have been extrapolated from studies in patients with Marfan syndrome, with mixed results.
A randomized trial28 found beta-blockers reduced expansion and even mortality in patients with Marfan syndrome with TAA, though this was not consistently reported in other studies. Nevertheless, beta-blockers are routinely prescribed in TAA, with adequate response represented by reduction in both blood pressure and heart rate, although they should not be used in those with significant aortic regurgitation.1
There is also some mixed evidence from randomized trials supporting the use of angiotensin II receptor blockers10,29 and angiotensin-converting enzyme inhibitors.30
The optimal blood pressure target remains controversial. The European guidelines advocate 140/90 mm Hg,1 while the American guidelines say 130/80 mm Hg in those with diabetes or chronic renal disease and 140/90 mm Hg in those without.2
Statins were seen in one study to reduce events in patients with abdominal aortic aneurysm but not those with TAA, so they are not routinely recommended for TAA.31 Nevertheless, many patients with TAA have concurrent atherosclerotic disease that would benefit from statin therapy.
HOW SHOULD TAA BE FIXED?
Interventions for TAA vary widely in complexity and are classified by location and by modality. Patients should be referred to a high-volume cardiac surgery center with aortic expertise for management to optimize outcomes.
Aneurysm of the ascending aorta mandates surgical repair with median sternotomy, cardiopulmonary bypass, and circulatory arrest.1,2 Considerations include the need to operate on the aortic valve (prosthetic valve composite graft or valve-sparing), aortic root (requiring coronary reimplantation), arch (complete or partial, brain protection with hypothermia, and perfusion method), and sometimes the descending aorta.
On the other hand, aneurysm in the descending aorta can be addressed with endovascular repair using percutaneous access in suitable anatomy, with or without arch-vessel transposition (debranching).1 The potential benefits are lower perioperative mortality risk and faster recovery than with surgery, although late complications such as graft leak, migration, and rupture can occur, and the durability is unknown.32,33
Surgery is the alternative option, with a higher threshold of aortic dimensions for intervention.1 It is done by thoracotomy and often without cardiopulmonary bypass while protecting the spinal cord. High surgical risk and restricted life expectancy favor endovascular repair, while genetic syndromes, peripheral vascular disease, and unfavorable anatomy favor surgery.1,2 A hybrid approach for surgery of the ascending aorta, arch, or both and endovascular repair for the descending aorta is sometimes considered in extensive TAA.
WHAT ELSE SHOULD BE MANAGED?
Management of TAA is multidisciplinary, with many aspects beyond medications and interventions. Patient education regarding warning symptoms and signs of TAA complications warranting immediate medical attention is important.1,2 Cardiovascular risk reduction is important, with nonpharmacologic measures such as healthy diet and smoking cessation, which have positive effects on blood pressure and lipids.
Exercise is controversial in patients with TAA. Although aerobic activity should probably be encouraged, weight-training activities such as heavy lifting should be avoided, particularly in those with genetic conditions such as Marfan syndrome or Loeys-Dietz syndrome.
There is also a weak association of acute aortic syndromes with fluoroquinolones, so avoidance may be considered.34
Counseling should be considered in patients with genetic conditions associated with TAA, women considering pregnancy or who are pregnant, and patients with indications for aortic interventions but who are being conservatively managed because of medical comorbidities and surgical risk.
In patients with genetic syndromes or bicuspid aortic valves who develop TAA, counseling and family screening starting with first-degree relatives (and beyond if multiple family members are positive) are important.1,2 Screening involves TTE, preferably CTA or MRA (used more because of no radiation), and genetic testing. If one or more first-degree relatives of a TAA patient are also found to have TAA, referral to a clinical geneticist for further testing and counseling is recommended. The implicated genes include FBN1 for Marfan syndrome; TGFBR1, TGFBR2, SMAD3, TGFB2, and TGFB3 for Loeys-Dietz syndrome, COL5A1, COL5A2, and COL3A1 for Ehlers-Danlos syndrome, and 45XO for Turner syndrome.1,35 Early detection of TAAs with surveillance and intervention have the potential to improve outcomes for patients and family members.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.