A 19-year-old woman with a history of mild intermittent asthma and regular tetrahydrocannabinol (THC) consumption by “vaping” and “dabbing” (inhaling vaporized, highly concentrated THC oil) presented with several days of fevers, dyspnea, and productive cough. She was febrile, tachycardic, and tachypneic. Laboratory testing revealed an elevated white blood cell count of 15.0 × 109/L (reference range 4.4–10.0 × 109/L) and a pro-calcitonin level of 0.3 ng/mL (reference range 0.1–0.5 ng/mL). Her initial chest radiograph was unremarkable.
She was treated with fluid resuscitation, nebulized albuterol, and antibiotics (ceftriax-one and azithromycin) but developed respiratory failure requiring oxygen by nasal cannula.
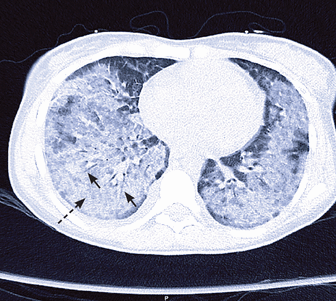
A repeat chest radiograph showed central consolidation (alveolar spaces filled with fluid) bilaterally in the midlungs and bases, with progression to widespread, patchy airspace disease (Figure 1). Computed tomography revealed bilateral dense consolidation with air bronchograms as well as peripheral sparing (Figure 2).
Chest radiograph showing widespread, patchy airspace opacification.
Computed tomography showing bilateral dense consolidation (dashed arrow) with air bronchograms (solid arrows) and peripheral sparing.
We initiated intravenous corticosteroid therapy and broadened her antibiotic coverage. Her respiratory failure necessitated oxygen supplementation by high-flow nasal cannula, and she was admitted to the intensive care unit.
Results from an extensive infectious disease workup were unremarkable, and her antibiotics were discontinued. Her acute lung injury was deemed secondary to chemical pneumonitis from inhalation of aerosolized THC.
After several days of corticosteroid therapy and supportive care, her respiratory status improved. She was counseled on THC cessation, prescribed oral corticosteroids, and discharged with a recommendation to follow up with a pulmonologist.
AN OUTBREAK OF LUNG INJURY
Among adult marijuana users, 33.7% report multiple methods of use, 19.4% report vaping, and 14.5% report dabbing, according to a 2016 national survey conducted by state health departments.1 Vaping is inhalation of aerosols produced by electronic cigarettes; dabbing refers to using high heat to vaporize highly concentrated THC in the form of butane hash oil, which is inhaled.
These THC-containing products have been implicated in an outbreak of e-cigarette or vaping product use-associated lung injury (EVALI), with more than 2,800 hospitalized cases and 68 deaths as of February 2020, according to data from the US Centers for Disease Prevention and Control2; 82% of the patients had used THC-containing products.
Does vitamin E acetate play a role?
The pathophysiology of EVALI may be mediated in part by vitamin E acetate, which became a popular additive to THC oil, given their comparable viscosities, around the same time as the recent outbreak. Vitamin E acetate is nearly ubiquitous in bronchoalveolar lavage samples taken from case patients but is absent in control groups.3 Its inhalation may precipitate lung injury by interfering with the ability of pulmonary surfactant to maintain surface tension or by producing ketene, a potentially noxious irritant. In dabbing, the THC product extracted from hash oil using liquid butane (butane hash oil) may degrade into pneumotoxic byproducts at high temperatures.4
SUSPECT IT IF PATIENTS REPORT VAPING
Suspect EVALI if patients report vaping or dabbing within 90 days of symptom onset, have pulmonary infiltrates on imaging, and lack evidence of an alternative cause. Patients commonly present with a gradual onset of constitutional, respiratory, or gastrointestinal symptoms.
Laboratory testing may reveal elevations in the white blood cell count, serum inflammatory markers, and aminotransferase levels.2,5 Infectious disease workup includes evaluation for viral, bacterial, endemic, and opportunistic pathogens, based on patient presentation and geographic prevalence.5
Differences from COVID-19 on imaging
Although radiographic findings may be nonspecific, certain imaging features can help differentiate EVALI from other novel lung disorders. Most cases of EVALI show basilar-predominant consolidation and ground-glass opacification that often spares the periphery, with interspersed segments of unaffected parenchyma.6
In comparison, the respiratory illness associated with coronavirus disease 2019 (COVID-19) typically causes ground-glass opacification in the acute phase, with subsequent development of septal thickening that may be interlobular (involving septa separating secondary pulmonary lobules) or intra-lobular (involving septa separating individual acini) and multifocal consolidation. Unlike EVALI, COVID-19 lesions most commonly have a peripheral or subpleural distribution.7,8
Despite these distinctions, serologic testing is required to confirm the diagnosis of COVID-19.
SUPPORTIVE CARE, CORTICOSTEROIDS, POSSIBLY ANTIBIOTICS
Management of EVALI focuses primarily on respiratory support, with consideration for empiric corticosteroid and antimicrobial therapy on a case-by-case basis. Significant clinical improvement has been reported with corticosteroid administration, likely due to suppression of the inflammatory response.5,9
Patients should be advised to discontinue substance use, and they may require outpatient follow-up.5
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.