A 59-year-old man with a history of hypertension presented with 2 months of progressive abdominal distention associated with abdominal pain and fatigue. Point-of-care ultrasonography revealed large-volume ascites. The patient’s abdominal swelling limited his activities of daily living and oral intake, resulting in hospital admission for new-onset ascites.
At admission, the patient’s temperature was 36.7°C (98.1°F), heart rate 82 beats per minute, respiratory rate 18 breaths per minute, and blood pressure 111/63 mm Hg. Results of pulmonary and cardiovascular examinations were normal, including the jugular venous pressure. Abdominal distention and bulging flanks were noted. However, there were no spider telangiectasias, palmar erythema, or distended abdominal veins. During abdominal paracentesis, the hospitalist procedure team noted that the ascitic fluid had a markedly viscous, jelly-like consistency (Figure 1).
Viscous ascites with the consistency of honey slowly oozes from a peritoneal catheter.
THE EVALUATION
Laboratory testing revealed the following:
Serum creatinine 0.86 mg/dL (reference range 0.50–1.39)
Albumin 3.8 g/dL (3.2–5.6)
Total bilirubin 0.8 mg/dL (< 1.2)
Direct bilirubin 0.3 mg/dL (< 0.4)
Alkaline phosphatase 69 U/L (35–137)
Aspartate aminotransferase 24 U/L (< 40)
Alanine aminotransferase 26 U/L (7–45)
International normalized ratio 1.09 (0.83–1.19)
Carcinoembryonic antigen 0.9 ng/mL (< 2.5)
Cancer antigen 19–9 46.8 U/mL (< 35 U/mL).
Ascitic fluid analysis showed the following:
White blood cells 2,016/μL (reference range < 300), with 43% neutrophils, 29% lymphocytes, 26% macrophages, 2% monocytes
Red blood cells 3,000/μL (0)
Culture: no bacterial growth
Chemistries including protein, albumin, triglycerides and amylase could not be completed due to fluid viscosity
Fluid cytology: positive for mucin and atypical malignant cells.
The fluid’s viscous appearance prompted consideration of pseudomyxoma peritonei (PMP) over more common causes of ascites due to the presence of portal hypertension. With a negative fluid culture, spontaneous bacterial peritonitis was unlikely in the absence of cirrhosis or portal hypertension despite the ascitic fluid neutrophil count being greater than 250 μL.
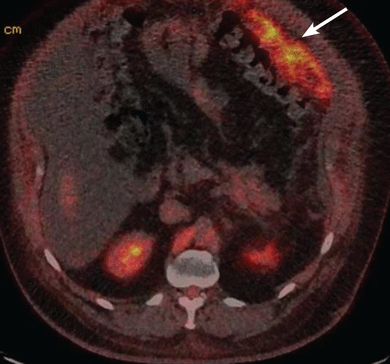
Magnetic resonance imaging of the abdomen revealed thickening (ie, “caking”) of the greater omentum, with new dilation of the pancreatic duct and a possible mass within the tail of the pancreas. Computed tomography (CT) revealed a normal appendix without another source for a primary tumor. Positron emission tomography/CT showed increased uptake along the greater omentum, suggestive of peritoneal carcinomatosis (Figure 2). Peritoneal biopsy revealed an immunohistochemical profile suggestive of a well-differentiated pancreaticobiliary adenocarcinoma. Colonoscopy and upper gastrointestinal endoscopy did not reveal another primary tumor. The combination of findings indicated either a ruptured intraductal papillary mucinous neoplasm (IPMN) complicated by invasive carcinoma or a pancreatic adenocarcinoma as the primary tumor.
Positron emission tomography/computed tomography performed after therapeutic paracentesis. There is significant hypermetabolic activity along the margin of the greater omentum (arrow) consistent with peritoneal carcinomatosis.
PSEUDOMYXOMA PERITONEI
Pseudomyxoma peritonei is a rare clinicopathologic syndrome with an incidence of 0.2 per 100,000 people per year.1 It is characterized by mucinous ascites associated with peritoneal and omental implants.1–3 Mucin-producing neoplastic cells rupture and seed the peritoneum.3 This results in a distinct gelatinous quality of the ascitic fluid. Patients with PMP typically present between ages 40 and 55, and the syndrome is often diagnosed as an incidental finding on imaging or during laparotomy.2 At later stages, PMP presents as new-onset ascites and abdominal distention.2,3
Low-grade or high-grade appendiceal mucinous neoplasms are the main cause of PMP.1 The primary differential diagnostic consideration is between an appendiceal mucinous neoplasm and peritoneal carcinomatosis in which invasive implants of mucinous adenocarcinoma result in mucinous ascites.3 Clinical outcomes for patients with peritoneal carcinomatosis are poor compared with those for patients with appendiceal mucinous neoplasms causing PMP.3 When confronted with PMP that is not of appendiceal origin, it is essential to determine the origin of the tumor, as neoplasms of the ovary, gallbladder, colon, urachus, and pancreas are associated with PMP.1 The relationship between IPMN and PMP is hypothesized based on case reports.4
TREATMENT AND FOLLOW-UP
The cornerstone of treatment for PMP is surgical debulking and hyperthermic intraperitoneal chemotherapy.2,3 Patients with unresectable disease and severe symptoms due to ascites may require serial paracenteses. Those with ascitic fluid viscosity that prohibits bedside drainage with traditional catheters may require referral to interventional radiology or possibly surgery for palliative laparoscopic evacuation of the ascites.5
Due to the rarity of PMP that is not of appendiceal origin, our patient’s treatment required a multidisciplinary approach to assess surgical risk and indications for systemic chemotherapy based on the tumor’s primary site of origin. The pathology suggested pancreatic ade-nocarcinoma, which prompted initial treatment with leucovorin, fluorouracil, irinotecan, and oxaliplatin (ie, the FOLFIRINOX regimen). Unfortunately, the patient experienced significant adverse effects and progressive functional decline. He declined additional surgical management, opting for palliative and comfort-based treatments, and died 8 months after the initial diagnosis.
New-onset ascites is a common patient presentation. Although PMP is rare, the unique, viscous quality of the ascitic fluid is easily recognizable. The presence of mucinous ascites on bedside paracentesis should prompt evaluation for an etiology of PMP including ascitic fluid cytology testing and cross-sectional abdominal imaging.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.