ABSTRACT
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of morbidity and mortality, with low-density lipoprotein (LDL) cholesterol being a causative risk factor. Though statins have a decades-long track record of efficacy and safety, nonstatin agents may be used to reduce LDL cholesterol as an adjunct or alternative to statin therapy. Several new nonstatin medications have been approved in recent years, with robust data from clinical trials supporting their use in atherosclerotic disease. This review addresses the indications, evidence, and important prescribing considerations for using nonstatin lipid-lowering therapy and proposes a practical approach for determining when to initiate nonstatin therapy.
The use of statins to reduce LDL cholesterol remains key to the prevention and treatment of ASCVD; target LDL cholesterol levels should be individualized based on cardiovascular risk profiles and shared decision-making.
Some patients are unwilling or unable to tolerate statin therapy, while others fail to achieve LDL cholesterol goals despite statin use. In such instances, clinicians may consider nonstatin therapy to lower LDL cholesterol.
Nonstatin lipid-lowering agents including ezetimibe, proprotein convertase subtilisin/kexin type 9 monoclonal antibodies, and bempedoic acid have been shown to reduce cardiovascular risk when given in conjunction with or in place of statins.
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of morbidity and mortality worldwide. ASCVD is a broad term that encompasses coronary heart disease (myocardial infarction or obstructive coronary artery disease), cerebrovascular disease (stroke, transient ischemic attack, or significant carotid artery stenosis), peripheral arterial disease (claudication or limb ischemia), aortic atherosclerotic disease, and prior coronary or arterial revascularization due to atherosclerosis.
It is now indisputable that low-density lipoprotein (LDL) cholesterol has a causal relationship to atherosclerosis, the process that underpins the development of clinical ASCVD.1 Lipoproteins are particles that transport fats throughout the body, and LDL specifically transports cholesterol. Standard lipid panels normally report the serum concentration of LDL cholesterol, ie, the amount of cholesterol being transported by LDL particles. In the past few decades, LDL cholesterol has emerged as a powerful predictor of cardiovascular risk and a determinant of target levels of lipid-lowering therapy. Encouragingly, a meta-analysis of 26 randomized trials found that each 1.0-mmol/L (18-mg/dL) decrease in LDL cholesterol resulted in a 22% relative risk reduction in major vascular events (P < .0001), further supporting the notion that lower is better when it comes to LDL cholesterol.2
For decades, the cornerstone of both prevention and treatment of ASCVD has been 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, better known as statins. By inhibiting the rate-limiting enzyme in cholesterol synthesis, statins increase cell-surface LDL receptor expression and clearance of LDL cholesterol from the bloodstream. The Scandinavian Simvastatin Survival Study,3 published in 1994, was the first to demonstrate that statins improve outcomes (reduction in cardiovascular mortality and major coronary events with simvastatin) in patients with coronary artery disease and hyperlipidemia.
Successive large-scale clinical trials over the next 3 decades added more and more supporting evidence. Today, statins are one of the most prescribed drugs in clinical practice. Given the overwhelming evidence of cardiovascular benefit conferred by these agents, clinicians should continue to prescribe statins at the maximum tolerated doses for appropriate patients.
While statin therapy has a long track record of safety and efficacy in treating ASCVD, there are instances in which nonstatin lipid-lowering therapies may be needed. These scenarios include patient unwillingness to take statins, intolerability of statin side effects, and failure to meet LDL cholesterol goals with statin therapy alone. An analysis of the Patient and Provider Assessment of Lipid Management registry found that more than 25% of adults meeting criteria for statin therapy were not taking one, largely because they were never offered a statin or because they were concerned about potential adverse effects.4 Nearly 55% of former statin users in the registry cited perceived side effects, most commonly muscle-related symptoms, as the primary reason for drug discontinuation.4 Similarly, a recent meta-analysis estimated between 5% and 17% of patients discontinue statins due to medication side effects, rates far higher than expected in clinical trials.5 These findings emphasize the need for ongoing patient education regarding statin use and for clinician familiarity with nonstatin therapies for lipid management.
This review simplifies the guidance on when to consider the addition of nonstatin therapy for LDL-lowering based on recent clinical trial data. We also aim to provide practical strategies that clinicians can use to determine the most appropriate nonstatin therapy as an adjunct to or in place of statins. Many of the recommendations in this review are based on the 2018 American College of Cardiology/American Heart Association cholesterol guidelines6 and the subsequent 2022 American College of Cardiology Expert Consensus Decision Pathway7 on the role of nonstatin therapies for lowering LDL cholesterol. We also include recently published outcome data from CLEAR (Cholesterol Lowering via Bempedoic Acid [ECT1002], an ACL [adenosine triphosphate-citrate lyase]-Inhibiting Regimen).8 This large double-blind, randomized controlled trial of 13,970 patients with statin intolerance were assigned to bempedoic acid or placebo, which has provided robust evidence for another nonstatin agent in the ever-changing landscape of LDL cholesterol management.
INDICATIONS AND GOALS FOR LIPID-LOWERING THERAPY
A holistic assessment of each patient’s cardiovascular risk and baseline lipid profile is essential for determining goal levels of LDL cholesterol. The 2018 American College of Cardiology/American Heart Association Task Force multisociety guideline6 on the management of blood cholesterol and the 2019 European Society of Cardiology/European Atherosclerosis Society guidelines9 for the management of dyslipidemias discuss in detail the indications and treatment goals for lipid-lowering therapy. Evidence-based indications for lipid-lowering therapy are divided into primary and secondary prevention of ASCVD, and recommendations for either moderate- or high-intensity statin therapy depend on estimated cardiovascular risk.
Patients treated for primary prevention include adults with LDL cholesterol of at least 190 mg/dL or patients between ages 40 and 75 who have diabetes or an estimated 10-year risk for ASCVD of least 7.5% (taking into consideration comorbidities and risk-enhancers).6,9 On the other hand, patients treated for secondary prevention have clinical manifestations of ASCVD—cardiovascular, cerebrovascular, or peripheral arterial disease—and are further subdivided into high-risk and very-high-risk categories. In patients for whom lipid-lowering therapy is indicated, the next decision is to what level the cholesterol—most commonly LDL cholesterol—should be lowered. Over the past decade, more and more data have supported the notion that lower is better regarding levels of atherogenic lipids.
The society guidelines noted indicators for efficacy and suggest that there is a relative target level of cholesterol reduction (ie, 30% to 49% reduction for moderate-intensity statins or ≥ 50% reduction for high-intensity statins from baseline LDL cholesterol) for patients treated with lipid-lowering therapy.6,7,9 One concern with this strategy from a practical perspective is that many patients have been on some form of LDL-lowering therapy, and a true “baseline” LDL level may not be available. Another issue with this approach is that patients with significant hypercholesteremia may have LDL cholesterol levels that remain substantially elevated even after relative reduction. Accordingly, most experts advocate for an absolute LDL cholesterol target alongside a relative reduction and consider the addition of nonstatin therapy when patients remain above goal despite maximally tolerated statin therapy. Absolute LDL cholesterol targets range from 55 mg/dL to 100 mg/dL depending on indication for therapy, overall cardiovascular risk, and patient goals of care.7
In recent years, several new nonstatin agents have been shown in clinical trials to both lower LDL cholesterol levels and reduce cardiovascular events in select patients (Table 1).7,8,10–27 It is worth noting that to date, no LDL-lowering nonstatin therapy has been shown to reduce all-cause or cardiovascular mortality. This may be due to underpowering, inadequate follow-up duration, or a true lack of mortality benefit in the era of goal-directed medical therapy. Regardless, reduction in rates of myocardial infarction, stroke, or coronary revascularization is very meaningful clinically. In the following section, we review nonstatin therapies that clinicians may consider as an adjunct or alternative to statins in select patients.
Main nonstatin lipid-lowering therapies
SPECIFIC NONSTATIN THERAPIES
Ezetimibe
Ezetimibe, US Food and Drug Administration (FDA)-approved in 2002, is the most prescribed non-statin agent for the treatment of hyperlipidemia.7 Ezetimibe blocks the Niemann-Pick C1-Like 1 protein and inhibits uptake of cholesterol in the small intestine, thereby reducing the absorption of dietary and biliary cholesterol.7 This subsequently promotes synthesis of hepatic LDL receptors, resulting in a reduction of serum LDL cholesterol.10 Ezetimibe is an oral medication and lowers serum LDL cholesterol by an additional 13% to 25% from baseline when added to statin therapy depending on statin intensity, or 15% to 19% when given as monotherapy compared with placebo.10,11 Ezetimibe is affordable and generally well-tolerated, with principal side effects of headache and upper respiratory tract symptoms occurring in 4% and 8% of patients.11 In addition, no dosage adjustments are required for individuals with hepatic or renal impairment.
The IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial),12 published in 2015, evaluated the effect of ezetimibe in combination with simvastatin, compared with simvastatin alone, in 18,144 patients with recent acute coronary syndrome. At 7 years, the rates for the primary end point (composite of death from cardiovascular disease, major coronary event, or nonfatal stroke) were 32.7% in the simvastatin-ezetimibe group and 34.7% in the simvastatin monotherapy group, with an absolute risk reduction of 2% and number needed to treat of 50 patients over 7 years to prevent 1 event. A later analysis found a high rate of subsequent events not included in the primary analysis—thus, the cardiovascular risk reduction from ezetimibe may be even greater than the original trial suggests.13
IMPROVE-IT firmly established the utility of ezetimibe, with other trials lending further support. Published in 2011, the SHARP (Study of Heart and Renal Protection) trial13,14 randomized patients with chronic kidney disease and without clinical ASCVD to receive ezetimibe with simvastatin or placebo. With a median 4.9-year follow-up, the study found that patients receiving simvastatin and ezetimibe had a lower incidence of the composite end point (myocardial infarction, coronary death, ischemic stroke, or any revascularization procedure) compared with those receiving placebo (11.3% vs 13.4%; absolute risk reduction 2.1%; number needed to treat 50),13,14 though there was limited analysis of the combination compared with simvastatin alone.
The more recent RACING (Randomised Comparison of Efficacy and Safety of Lipid Lowering With Statin Monotherapy Versus Statin–Ezetimibe Combination for High-Risk Cardiovascular Disease) trial,15 which enrolled 3,780 patients and was published in 2022, demonstrated that the combination of moderate-intensity rosuvastatin and ezetimibe was noninferior to high-intensity rosuvastatin therapy for the composite primary end-point events (cardiovascular death, major cardiovascular events, nonfatal stroke) over a 3-year period. Also notable was the fact that patients receiving combination therapy achieved lower levels of LDL cholesterol and a lower incidence of drug intolerance (4.8% vs 8.2%). These clinical trials have demonstrated ezetimibe’s efficacy, safety, and impact on cardiovascular outcomes across a wide spectrum of patients receiving lipid-lowering therapy.
PCSK9 monoclonal antibodies
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a protein produced primarily by hepatocytes that circulates in the plasma and binds to LDL receptors, triggering a signaling cascade resulting in lysosomal degradation of the LDL receptors and decreased LDL cholesterol clearance.16 These fully human monoclonal antibodies bind free plasma PCSK9, preventing PCSK9 interaction with the LDL receptor. This results in increased LDL receptor recycling within hepatocytes and increased clearance of circulating LDL cholesterol. PCSK9 monoclonal antibodies are administered subcutaneously, usually at 2- or 4-week intervals, and tend to lower LDL cholesterol by approximately 50% when given alone, and by approximately 70% in patients already on statin therapy.16
Two PCSK9 antibodies—alirocumab and evolocumab—were FDA-approved in 2015.7,17–19 The GAUSS-3 (Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects 3) trial17 randomized patients with statin-intolerance to evolocumab or ezetimibe and demonstrated a far more potent LDL cholesterol reduction in patients receiving evolocumab (52.8% vs 16.7%).
In the following years, 2 randomized placebo-controlled trials confirmed the efficacy of PCSK9 monoclonal antibodies in reducing cardiovascular events.18,19 The FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial,18 published in 2017, randomized 27,564 patients with clinical ASCVD and LDL cholesterol levels greater than or equal to 70 mg/dL despite statin therapy to the addition of evolocumab or placebo. At a median follow-up of 2.2 years, evolocumab reduced the risk of the primary end point (composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization) compared with placebo (9.8% vs 11.3%; absolute risk reduction 1.5% with number needed to treat 74). The only notable difference in adverse events between groups was a higher rate of injection-site reactions in the evolocumab group (2.1% vs 1.6%).
The ODYSSEY (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) Outcomes trial19 evaluated whether alirocumab reduced the risk of recurrent ischemic cardiovascular events in 18,924 patients with recent acute coronary syndrome and cholesterol levels above goal despite maximally tolerated statin therapy. At a median follow-up of 2.8 years, the alirocumab group had a lower incidence of the composite primary end point (death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) compared with placebo (9.5% vs 11.1%; absolute risk reduction 1.6% and nearly identical to the outcome observed in the FOURIER trial). The ODYSSEY trial had a similarly high rate of local injection-site reactions in the PCSK9 monoclonal antibodies group.
The advent of PCSK9 monoclonal antibodies allowed for the reduction of LDL cholesterol to levels rarely achieved with conventional lipid-lowering therapy, along with an excellent safety profile. The corresponding impact on cardiovascular outcomes gave further credence to the notion that lowering LDL cholesterol levels beyond what is attainable with statins is possible and offers incremental cardiovascular benefit.
Bempedoic acid
Bempedoic acid is an oral medication that inhibits adenosine triphosphate citrate lyase, an enzyme in the cholesterol biosynthesis pathway upstream of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (the target of statins).7,8,20,21 Like the effect of statins, inhibition of cholesterol synthesis results in increased LDL cholesterol receptor expression and increased clearance of serum LDL cholesterol. Notably, the enzyme required to activate bempedoic acid is present in hepatocytes but absent in skeletal muscle, resulting in far lower concern for statin-associated muscle symptoms compared with statin therapy. Bempedoic acid lowers LDL cholesterol by 17.2% to 26.5% as monotherapy, by 16.5% to 18% when added to a background of statin therapy, and by up to 50% when given as a fixed-dose combination with ezetimibe.20,21
In the recently published CLEAR Outcomes trial,8 13,970 patients with a prior cardiovascular event or at high risk for ASCVD and unable to tolerate more than a very low dose of a statin (22.7% of patients were taking a low-dose statin and 11.5% were taking ezetimibe) were randomized to receive oral bempedoic acid or placebo. In this trial, bempedoic acid lowered LDL cholesterol by about 20% from baseline, and patients receiving bempedoic acid had a 21.6% greater reduction in high-sensitivity C-reactive protein levels compared with patients receiving placebo. At a median follow-up of 40.6 months, patients receiving bempedoic acid had a lower incidence of the composite primary end point (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization) compared with placebo (11.7% vs 13.3%; absolute risk reduction 1.6% with number needed to treat of approximately 63.6).8,28 Bempedoic acid was well-tolerated, with rates of discontinuation in the bempedoic acid arm similar to those with placebo. Side effects occurring more frequently in patients receiving bempedoic acid compared with placebo included elevated liver aminotransferase levels (4.5% vs 3%), renal injury (11.5% vs 8.6%), gout (3.1% vs 2.1%), and cholelithiasis (2.2% vs 1.2%).8 It is important to note that in the CLEAR Outcomes trial,8 bempedoic acid was given in place of statin therapy (or with a very low average daily statin dose), not as adjunct treatment. This is in contrast to the landmark trials of other nonstatin therapies, which were generally added to a background of maximally tolerated statin therapy. This trial specifically targeted patients who could not or would not tolerate statin therapy. Patients and their physicians specifically documented the inability or refusal to take a higher dose of statin despite understanding the benefit of statins.8 While bempedoic acid lacks outcomes data when given alongside high-intensity statins, it is encouraging that patients who do not tolerate statins have another available therapy that lowers LDL cholesterol and reduces cardiovascular risk.
As mentioned, bempedoic acid is also available in combination with ezetimibe (1 tablet containing 180 mg of bempedoic acid and 10 mg ezetimibe). This combination is FDA-approved to lower LDL cholesterol levels in adults with ASCVD or familial hyper-cholesterolemia.7,22 A phase 3 clinical trial found that after 12 weeks of treatment, this combination reduced LDL cholesterol levels by 36.2%, a greater reduction than that with either bempedoic acid (17.2%) or ezetimibe (23.2%) alone.22 In addition, a recent phase 2 study demonstrated that combination bempedoic acid, ezetimibe, and atorvastatin triple therapy was generally well-tolerated and lowered LDL cholesterol levels by 63.6% compared with placebo, with more than 90% of patients achieving LDL cholesterol concentrations below 70 mg/dL.29 While there are as yet no cardiovascular outcome data specific to this therapy, the significant reduction in LDL levels from synergistic oral therapy is encouraging.
Inclisiran
Beyond monoclonal antibodies for PCSK9-lowering, there have been recent advances in using small interfering RNA molecules (siRNA) to reduce PCSK9 translation at the cellular level. The siRNA molecules engage the natural pathway of RNA interference and lead to the degradation of PCSK9 mRNA, resulting in decreased production of the PCSK9 protein.23 One such siRNA targeting the PCSK9 protein, inclisiran, was granted FDA approval in December 2021 as an adjunct for adults with clinical ASCVD or familial hypercholesteremia who require lowering of LDL cholesterol beyond what is achieved with statins.7
Phase 3 clinical trials published in 2020 (A Randomized Trial Assessing the Effects of Inclisiran on Clinical Outcomes Among People With Cardiovascular Disease [ORION] 9, 10, and 11) demonstrated that inclisiran, administered twice yearly as an injection, was well-tolerated without any major adverse events.24,25 Patients receiving inclisiran had a 39.7% to 52.3% reduction of LDL cholesterol on top of statin therapy.25 It is worth noting, however, that inclisiran appears to be less efficacious at lowering LDL cholesterol than PCSK9 monoclonal antibodies.26
In an extension study of ORION-1,26 92 patients originally assigned to placebo were treated with twice-monthly evolocumab for 1 year and subsequently transitioned to twice-yearly inclisiran. Treatment with evolocumab lowered LDL cholesterol by 61% followed by a time-averaged LDL cholesterol reduction of 45% over 3 years after switching to inclisiran.26 Though the early data on LDL cholesterol reduction and drug safety appear promising, larger trials examining the impact of siRNA-based therapies on cardiovascular risk reduction are ongoing, and effects of inclisiran on cardiovascular outcomes remain undetermined. There are currently 2 ongoing trials—ORION-4 and VIC-TORION-2P Prevent (A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial, Assessing the Impact of Inclisiran on Major Adverse Cardiovascular Events in Participants With Established Cardiovascular Disease) that aim to determine whether inclisiran will impact cardiovascular morbidity or mortality for primary and secondary prevention.
THERAPIES NOT ROUTINELY RECOMMENDED FOR LIPID MANAGEMENT
Dietary supplements
Nearly every clinician who prescribes lipid-lowering pharmacotherapy has been asked about the use of dietary supplements to lower LDL cholesterol. Of the many available supplements, red yeast rice and plant sterols have the most data supporting lipid-lowering, with various studies reporting LDL cholesterol reductions on the order of 10% to 25%.30,31 In fact, plant sterols are endorsed as an option to lower blood cholesterol levels in the 2019 revision of the European Society of Cardiology/European Atherosclerosis Society dyslipidemia guideline.9 A principal problem, however, is that most supplements are not FDA-regulated, so different manufacturers or formulations may have varying efficacy. Further, there is a dearth of quality data on the effect of these supplements on cardiovascular health.
The recently published Supplements, Placebo, or Rosuvastatin Study32 compared the efficacy of common supplements that have been purported to lower lipid levels on lowering the LDL cholesterol concentration.32 This prospective, single-blind clinical trial randomized 190 patients without evidence of clinical ASCVD but with an increased 10-year ASCVD risk and LDL cholesterol of 70 to 189 mg/dL to receive rosuvastatin 5 mg daily, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, or red yeast rice. After 28 days, rosuvastatin decreased LDL cholesterol levels by 35.2%, while none of the dietary supplements demonstrated a significant decrease in LDL cholesterol compared with placebo. Though this trial did not assess cardiovascular outcomes, it provides evidence that the studied supplements—often promoted for cholesterol-lowering benefits—do not significantly impact levels of atherogenic lipids.
Bile acid sequestrants
Bile acid sequestrants, such as cholestyramine, colesevelam, or colestipol, were one of the first classes of lipid-lowering therapies.7,27 These nonabsorbed polymers bind intestinal bile acids and impede their reabsorption, leading to a decrease in the bile acid pool and concurrent increase in the conversion of cholesterol to bile acids. The net effect is a modest reduction of LDL cholesterol, with possible increase in serum triglyceride concentrations. On average, bile acid sequestrants were shown to reduce LDL cholesterol by about 15% as monotherapy and an additional 10% to 16% in combination with statin therapy.
The Lipid Research Clinics Coronary Primary Prevention trial,27 published in 1984, randomized 3,806 asymptomatic men with primary hypercholesterolemia to cholestyramine compared with placebo for an average of 7.4 years. Cumulative incidence of the primary end point (definite coronary heart disease death and/or definite nonfatal myocardial infarction) was 7% in the cholestyramine group compared with 8.6% in those receiving placebo (relative risk reduction of 19% and absolute risk reduction of 1.6%).27 Though this study showed an effect on cardiovascular outcomes, the use of bile acid sequestrants is limited by drug-drug interactions (often decreasing absorption of other medications) and frequently intolerable gastrointestinal side effects, including nausea, constipation, and dyspepsia. In light of the cardiovascular outcomes data for both statins and recent nonstatin therapies, the clinical utility of bile acid sequestrants continues to diminish.
Niacin and fibrates
Niacin and fibrates (fenofibrate or gemfibrozil) are prescribed primarily as triglyceride-lowering drugs, though they may also mildly lower LDL cholesterol levels.33 The effects of fibrates on LDL cholesterol are quite minimal, and more importantly, randomized trials have not reliably shown these therapies to reduce cardiovascular risk. Some older data suggested that fibrates may be beneficial in patients with high triglyceride or low high-density lipoprotein cholesterol levels, though the effect is more modest than statins, and the combination of fibrates and statins often results in significant myalgias.
Despite lowering LDL cholesterol, niacin is poorly tolerated with significant side effects, and more importantly has failed to demonstrate a benefit for cardiovascular outcomes in the era of statins.34 While niacin and fibrates may have a niche in carefully selected patients with very high triglycerides, neither is currently recommended as an alternative or adjunct to statin therapy for lowering LDL cholesterol.
THERAPIES SPECIFIC TO PATIENTS WITH FAMILIAL HYPERCHOLESTEROLEMIA
Familial hypercholesterolemia is an inherited disorder that results in very high levels of LDL cholesterol and an increased risk of premature ASCVD. Clinical features differ depending on whether one or both alleles are affected. While homozygous familial hypercholesterolemia is very rare, heterozygous familial hypercholesterolemia is the most common monogenic autosomal dominant disorder, affecting 1 in 250 individuals.24,35 Patients with heterozygous familial hypercholesterolemia are nearly always treated with lipid-lowering therapy and will often require additional therapies beyond high-intensity statins to lower LDL cholesterol.36 Patients with homozygous familial hypercholesterolemia will nearly always require nonstatin therapies, and there are specific therapies, such as lomitapide (a microsomal triglyceride transfer protein inhibitor, necessary for very low LDL assembly and secretion) and evinacumab (a monoclonal antibody against angiopoietin-like protein 3, a regulator of lipoprotein metabolism), approved specifically for these patients.36 These therapies are not currently approved for nonhomozygous familial hypercholesterolemia patients and are given under the direction of a lipid specialist; they are thus beyond the scope of this review.
CHOICE OF NONSTATIN THERAPY: A PRACTICAL APPROACH
Once patient and clinician have decided to initiate nonstatin therapy, there are multiple factors that should be considered when choosing the agent. First, patients should preferentially be prescribed therapies that have been shown not only to lower LDL cholesterol, but also to reduce ASCVD risk. Therapies with high-quality evidence for reducing cardiovascular events include ezetimibe, bempedoic acid, and PCSK9 monoclonal antibodies (landmark trials detailed in Table 2).8,12,18,19
Clinical trials of nonstatin therapy
There is also biological plausibility for ASCVD risk-reduction benefit with bempedoic acidezetimibe combination therapy and inclisiran, though these therapies lack outcome data currently. Other important considerations for patients may include efficacy of LDL cholesterol-lowering, route of administration (oral or subcutaneous injection), cost (insurance plan coverage, availability of assistance programs, or need for prior authorization), and attention to drug-drug interactions and side effects of each agent.37 Given the number of patients requiring lipid-lowering therapy, with multiple agents to choose from and varying recommendations from major societal guidelines, a simplified approach is needed.
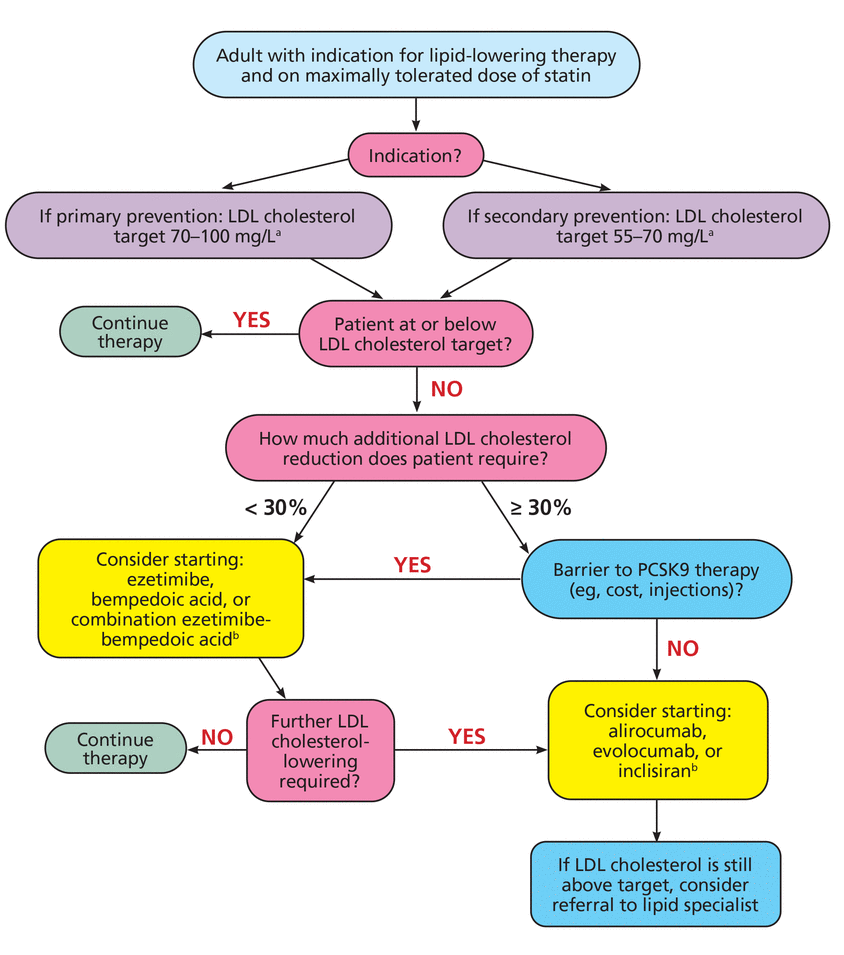
We propose an algorithmic approach for the addition of nonstatin therapy (Figure 1). From a practical standpoint, we categorize LDL cholesterol goals by whether patients are being treated for primary prevention (goal LDL cholesterol 70 to 100 mg/dL) or secondary prevention (goal LDL cholesterol 55 to 70 mg/dL), with the understanding that patients and clinicians will modify LDL cholesterol goals based on patient cardiovascular risk profile and the desire for more-aggressive rather than less-aggressive cholesterol reduction. We propose to subsequently stratify the choice of nonstatin therapy based on patient LDL cholesterol level at the time of initiation of therapy, which informs how much additional LDL cholesterol-lowering is required.
Practical approach to the addition of nonstatin therapy.
aIndividual LDL-cholesterol target based on patient risk profile.
bNo current cardiovascular outcome data.
LDL = low-density lipoprotein; PCSK9 = proprotein convertase subtilisin/kexin type 9
In patients who require LDL cholesterol-lowering of at least 30% from current levels, upfront therapy with a PCSK9 monoclonal antibody (alirocumab or evolocumab) is reasonable, assuming the patient does not have financial coverage barriers and can tolerate subcutaneous injections. Inclisiran is a possible alternative for these patients, though cardiovascular outcome data are not yet available. For patients who have barriers to PCSK9-inhibiting therapies or require less than 30% LDL cholesterol-lowering, ezetimibe or bempedoic acid are evidence-based oral options with modest impact on LDL cholesterol levels, though greater reduction can be achieved with combination therapy.
FUTURE DIRECTIONS
ASCVD remains the world’s leading cause of death despite advances in our understanding of the disease process. Statin therapy has been revolutionary in improving cardiovascular outcomes, particularly for high-risk patients, but there remains a need for other forms of lipid-lowering therapy as an adjunct or alternative to statins. Landmark clinical trials have cemented ezetimibe, PCSK9 monoclonal antibodies, and bempedoic acid as nonstatin agents that both lower LDL cholesterol levels and provide cardiovascular benefit to patients, with a large-scale outcome trial currently testing the efficacy of inclisiran.
Additional novel therapies to lower LDL cholesterol are entering clinical development, including recently completed phase 2b studies of an oral macrocyclic peptide PCSK9 inhibitor (MK-0616) and a liver-targeted antisense oligonucleotide that inhibits PCSK9 expression (AZD8233).38,39 Other regulators of LDL cholesterol levels, such as angiopoietin-like protein 3, apolipoprotein C-III, and cholesteryl ester transfer protein have also emerged as promising targets for lipid-lowering drug development.40,41 There is no doubt that the landscape of nonstatin therapies will continue to evolve in coming years, with each therapy having unique indications, advantages, disadvantages, and evidence base, and ultimately providing patients more therapeutic options for reducing cardiovascular risk.
DISCLOSURES
Dr. Cho has disclosed consulting for AstraZeneca, Esperion, and Merck; research: PI for AstraZeneca and Novartis; steering committee for CLEAR outcomes for Esperion. The other author reports no relevant financial relationships which, in the context of his contributions, could be perceived as a potential conflict of interest.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.