Myocardial infarction with nonobstructive coronary arteries (MINOCA) was first documented more than 70 years ago based on autopsy data describing myocardial necrosis in the absence of obstructive epicardial coronary disease.1
MINOCA accounts for approximately 5% to 15% of all myocardial infarctions, yet it remains underappreciated and often misdiagnosed, leading to gaps in appropriate management and treatment.2 In 2019, the American Heart Association (AHA) published a scientific statement3 specifically addressing this issue, offering a comprehensive framework for diagnosing and managing patients who experience myocardial infarction without significant coronary artery obstruction.
In this issue of the Journal, Buda et al4 provide a critical appraisal and a clinical workflow perspective of the AHA statement. Their article is clinician-friendly and aims to simplify the algorithm outlined in the AHA statement while explaining the significance of each of its components. They critique some of the content of the statement, emphasizing that many of the advanced imaging techniques advocated by the AHA algorithm are not readily available in smaller medical centers. While the AHA authors seem to consider practicing cardiologists as the primary audience, Buda et al aim to bring the workup of MINOCA to the internist or hospitalist, who are often confronted with these patients.
We agree with the authors of both the AHA statement3 and this review article4 that a thorough understanding of the definition and causes of MINOCA and its diagnosis is crucial in contemporary practice, particularly with the advent of high-sensitivity troponin assays and multimodality imaging techniques.
INVASIVE VS NONINVASIVE IMAGING
As highlighted by Buda et al,4 MINOCA disproportionately affects women of childbearing age who do not have the traditional risk factors for atherosclerotic cardiovascular disease. The authors appropriately advocate for a comprehensive and holistic patient assessment to rule out alternative and competing diagnoses. Once MINOCA is established as the working diagnosis, they recommend a cardiac-focused workup, including Transthoracic echocardiography, cardiac magnetic resonance imaging (MRI), coronary angiography, or combinations of these tests.
Realistically, to diagnose MINOCA, one must confirm there are no epicardial obstructive coronary lesions, which can only be done by performing invasive angiography or coronary computed tomography (CT) angiography. Because these patients have evidence of myonecrosis (elevated troponin), most clinicians and workflow patterns would steer them toward invasive angiography. While it is noted that cardiac MRI and transthoracic echocardiography are done before angiography in routine clinical settings, urgent or emergent coronary angiography often takes precedence during episodes of acute coronary syndrome, as most institutions have well-established protocols for activating the catheterization laboratory but lack algorithms for urgent noninvasive imaging.
MINOCA IS A WORKING DIAGNOSIS THAT NEEDS TO BE NARROWED DOWN
The authors agree with the AHA statement that MINOCA is a working diagnosis that needs to be refined or narrowed down to a specific etiology to initiate appropriate treatment. They classify the causes of MINOCA into atherosclerotic conditions, such as plaque disruption, and nonatherosclerotic conditions, including spontaneous coronary artery dissection, coronary thromboembolism, and coronary vasospasm.
Although we appreciate the concept of keeping it simple and using available resources, clinching the specific etiology of MINOCA frequently requires advanced techniques beyond angiography. The most fruitful of them are cardiac MRI performed in a timely manner, intravascular imaging performed in a timely manner, and assessment of microvascular and vasospastic disorders of the coronary arteries.
CARDIAC MRI CAN POINT TO ETIOLOGY
Buda et al4 agree that cardiac MRI is quite helpful in defining the etiology of MINOCA. They argue that it should precede invasive testing, which is a valid argument, with exceptions that we discussed earlier.
The advantage of MRI over simpler cardiac imaging modalities such as echocardiography is that it provides insight into the site and distribution of myocardial injury, as defined by late gadolinium enhancement, myocardial edema, or both (Figure 1). The distribution of such injury can point to its etiology: epicardial or midmyocardial enhancement is likely caused by myocarditis, whereas ischemic injury is always subendocardial and frequently follows a specific regional anatomic pattern. That pattern can also help in selecting the targets of intracoronary imaging and interrogation when invasive testing ensues.
Cardiac magnetic resonance imaging can reliably distinguish between myocarditis and coronary ischemic events in patients presenting with myocardial infarction with nonobstructive coronary arteries. Left panel, gadolinium enhancement (arrows) in the middle of the myocardium in the septum and below the epicardium in the lateral wall is a distribution pattern consistent with inflammatory disease or myocarditis. Right panel, enhancement (arrows) beneath the endocardium along the anterior septum and the anterior apex is a distribution consistent with an acute coronary injury likely originating from disease of the left anterior descending artery.
In a large single-center series of 719 patients with suspected acute coronary syndrome and nonobstructive coronary arteries,5 the MRI-based diagnosis was myocardial infarction in 26%, myocarditis in 26%, stress cardiomyopathy in 12%, and other cardiomyopathy in 10%; the remaining 26% had normal or nonspecific scans. Importantly, imaging within 14 days of the event was an independent predictor of reaching a diagnosis, as late gadolinium enhancement and edema fade over time.
ADVANTAGES OF INVASIVE ANGIOGRAPHY AND INTRAVASCULAR IMAGING
As mentioned above, angiography is an essential step in diagnosing MINOCA. Invasive angiography has some advantages over CT angiography, which is typically done in more stable patients rather than ones presenting with abnormal cardiac biomarkers. In addition, CT angiography may lack the resolution to identify subtle plaque disruptions such as erosion. And spontaneous coronary artery dissection, which typically affects smaller and tortuous vessels, is difficult to diagnose with certainty on CT angiography.
Invasive angiography also lets you perform Intracoronary imaging with either intravascular ultrasonography or optical coherence tomography. Atherosclerotic plaque disruption can result in more typical obstructive lesions and acute coronary syndromes, but in MINOCA, such disruption may be too subtle to define angiographically. Intracoronary imaging is crucial in identifying plaque rupture, plaque erosion, or eruptive calcified nodules—the 3 major pathways leading to myonecrosis.
While Buda et al4 say that intravascular ultrasonography may be good enough and that optical coherence tomography is not readily available in many institutions, these modalities are not equivalent. Optical coherence tomography has much higher resolution, about 10-fold that of intravascular ultrasonography, thus providing a fair chance of detecting any form of plaque disruption with higher sensitivity and specificity (Figure 2, Figure 3). Additionally, optical coherence tomography is much more suited to define intraluminal thrombosis compared with intravascular ultrasonography, as the echo density of an adherent or layered thrombus is frequently indistinguishable from that of a heterogeneous plaque.
Optical coherence tomography can define the underlying pathophysiology of the coronary event in myocardial infarction with nonobstructive coronary arteries. (A) Relatively mild angiographic disease of the proximal left anterior descending artery (arrow) in a patient with acute chest pain, abnormal biomarkers, and anterior T-wave inversions. (B) Optical coherence tomography demonstrates significant luminal narrowing and intraluminal thrombosis (asterisk). In that frame, the thrombus is shielding underlying plaque structure, but a few millimeters distally (C), the thrombus is still apparent and there is a disruption of the underlying intima. (D) Further distally, some thrombus is adherent, and there is an ulcer crater (X) after release of plaque content downstream.
Optical coherence tomography can define subtle findings beyond the resolution of angiography. In the left panel, angiography in a patient with myocardial infarction with nonobstructive coronary arteries shows minimal haziness in the proximal circumflex artery (arrow). In the right panel, optical coherence tomography demonstrates a small thrombus with intact underlying plaque, representing plaque erosion.
But anatomic evidence of plaque disruption may change over time. A small thrombus on top of an eroded plaque, or an even larger thrombus resulting from plaque rupture, will embolize downstream or be eliminated by intrinsic fibrinolytic mechanisms. Therefore, intracoronary imaging should be considered in the acute phase, ideally at the time of the initial angiogram, to maximize the chances of identifying culprit anatomic findings.
BEYOND IMAGING: TESTING FOR VASOSPASM AND MICROVASCULAR DYSFUNCTION
Although coronary vasospasm is not necessarily associated with obstructive lesions, it is essentially caused by coronary endothelial dysfunction and commonly associated with a degree of atherosclerosis. Non–endothelial-dependent coronary microvascular dysfunction is another important category of coronary disease that is not necessarily associated with obstructive lesions, but is a form of coronary disease nonetheless. In fact, microvascular dysfunction is grossly underdiagnosed and associated with major adverse cardiovascular events and worse medium- and long-term prognosis.6–8
The search for causes of MINOCA should include a detailed assessment of microvascular disorders with their various endotypes.9 Endothelial-dependent vasomotor disorders can be assessed using intracoronary acetylcholine provocation (Figure 4). Clinicians may elect to postpone provocative testing until after the acute phase, but unfortunately that requires an additional invasive procedure because there are no well-established noninvasive tests for coronary spasm.
Provocative acetylcholine testing for coronary spasm. In the left panel, a young female patient with a long history of smoking presenting with myocardial infarction with nonobstructive coronary arteries has mild luminal irregularities in the left anterior descending artery. The right panel shows that intracoronary injection of escalating doses of acetylcholine provokes anginal symptoms, ST depression in precordial leads, and epicardial spasm in the mid segment of the anterior descending artery (arrows). Calcium channel blockers, nitrates, or both can be used to control spasm and vasospastic angina, and aggressive risk factor control is also needed.
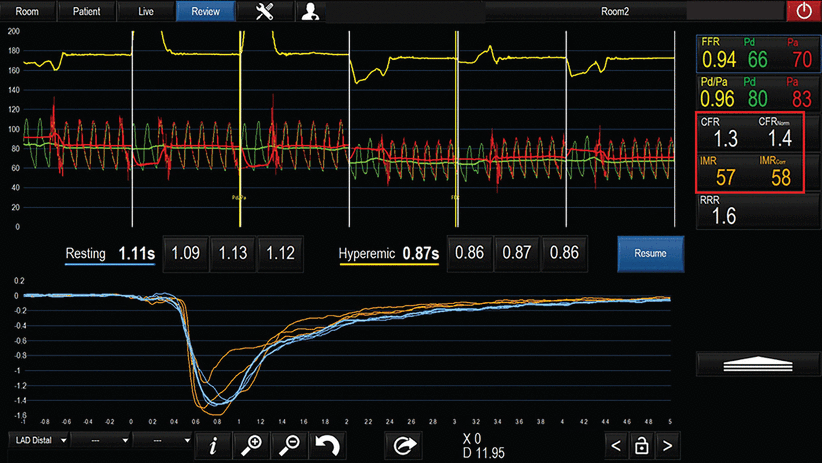
Testing for non–endothelial-dependent microvascular dysfunction involves assessing coronary flow reserve and microvascular resistance. This can be done in the catheterization laboratory using bolus or continuous thermodilution—injecting or infusing saline that is colder than body temperature.10 The resulting changes in pressure and temperature in the coronary arteries are used to calculate the coronary flow at rest; then hyperemia is induced using intravenous adenosine or intracoronary saline infusion, flow is measured again, and from these numbers coronary flow reserve is calculated. The pressure and temperature changes are also integrated to calculate microvascular resistance, independent of the impact of epicardial disease, if any (Figure 5).
Microvascular testing using thermodilution for assessment of coronary flow reserve (CFR). A patient presenting with myocardial infarction with nonobstructive coronary arteries and giving a history of exertional angina has no evidence of obstructive lesions. Thermodilution assessment based on transit time at rest and with hyperemia reveals abnormally low CFR and a high index of microcirculatory resistance (IMR; abnormal values in red box). Microvascular angina is diagnosed, and the patient can be treated with beta-blockers and ranolazine in addition to risk factor management.
FFR = fractional flow reserve; Pd = pressure measured distal to the stenosis; Pa = aortic pressure; RRR = resistive reserve ratio
Positron-emission tomography to measure myocardial blood flow during stress is a well-established, noninvasive tool for evaluating coronary microvascular dysfunction. While not mentioned by Buda et al,4 it can be considered in the diagnostic process of more-stable MINOCA patients as it has diagnostic and prognostic relevance.11
TREATMENT SHOULD BE INDIVIDUALIZED
Patients with MINOCA are a highly heterogeneous group, and their management should be tailored to the individual rather than a one-size-fits-all approach. The diagnosis of myocarditis or stress cardiomyopathy leads to an entirely different treatment algorithm than an ischemic etiology, whether epicardial or microvascular.
The authors4 propose treating patients with presumed coronary vasospasm using calcium channel blockers or nitrates without prior diagnostic testing, arguing that this treatment carries minimal risk. However, both American3 and European12 guidelines for management of chest pain recommend testing for coronary vasospasm and microvascular dysfunction before initiating treatment. Randomized trials in MINOCA patients demonstrated that treatment based on the specific endotype of microvascular disorder is superior to empiric treatment in terms of symptom relief and improvement of quality of life.13 Even experienced cardiologists frequently miss the true endotype based on clinical assessment and nonspecific tests.14
NOVEL TOOLS ARE IMPROVING DIAGNOSIS AND TREATMENT
MINOCA is an umbrella term that encompasses a number of disparate diagnoses. It is important to consider the spectrum of differential diagnoses until a true coronary etiology can be identified. Advances in cardiac and coronary imaging and physiologic assessment now allow for a thorough and accurate workup, which underlie the appropriate therapy and outcome. While clinical assessment and basic tests are irreplacable, novel tools have undeniably improved the yield of MINOCA workups and targeted therapies.
DISCLOSURES
Dr. Ziada has disclosed teaching and speaking for Abbott Vascular. Dr. Zghyer reports no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2024 The Cleveland Clinic Foundation. All Rights Reserved.